How to produce a superb combined IQ OQ PQ Protocol
Computer: Software: HVAC: Spreadsheet: Equip: Climatic Zone: Quality Steam
Understanding How to produce a superb combined IQ OQ PQ Protocol that is intuitive to edit, easy to use and compliant with 21 CFR Part 820/210/211/11 and includes a lead through completion SOP. Have you got it now?
The IQ OQ PQ Template design for combined protocol templates was originated by Validation Online, in response to several hundred reader suggestions we received in our ‘Suggestions Section’, over 14 years ago. It was specifically designed to make it the preferred choice for Process and Laboratory stand alone equipment. It is interactive. It has also found favor with project and line managers along with most validation consultants.
The Installation Qualification Section of combination IQ OQ PQ establishes documented qualification that key aspects of the equipment adhere to approved design intentions and that the recommendations of the manufacturer have been suitably considered. The Operational Qualification (OQ) "section 2" of combination IQ OQ PQ Template establishes that there is documented verification that the installed system functions as specified and that there is sufficient documentary qualification evidence to demonstrate this. The Performance Qualification (PQ) "section 3" of combination IQ OQ PQ template gives documented verification that the equipment performance in its normal operating environment is consistently "exactly as specified" in the User Requirements Specification (URS).
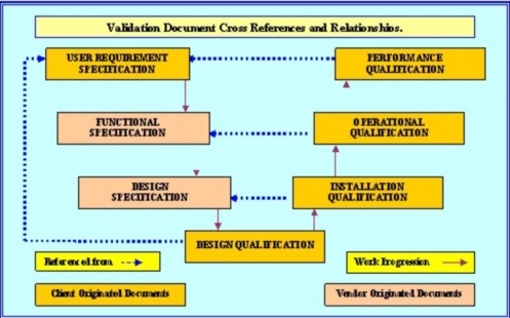
Interactivity Between Mandated Documents
Introduction to IQ OQ PQ Template
These IQ OQ PQ template contains over seventeen fully detailed qualification test scripts along with the methodology for twenty more compliance tests and inspections. It has been collated from the three individual IQ OQ PQ template and incorporates sufficient inspections and test stages to ensure that the equipment under qualification has been properly installed, operates and performs in a manner that satisfies all the appropriate requirements as detailed in the relevant User Requirements Specification (URS), cGMP’s and your company practices and procedure rulings.
All companies carryout routine calendar based qualification and re-qualification functional testing of stand-alone laboratory type equipment. Unfortunately most of this testing is documented in a manner that is not compatible with basic FDA, WHO & EU validation qualification protocol standards. If a little time was spent on revamping these test documents standards, (changing the templates would only have to be done once) a format could be used that would enable all such testing to be included in any qualification online requirement.
Since regulators already accept the concept that unnecessary retesting should be avoided whenever possible. It follows that since all other tests in our protocols are fully written up, if the routine Functional Test Specification (FTS) was of the right format then a recently executed routine or commissioning test could be reviewed and commented on in any validation online protocols, as an alternative to repeating all the IQ OQ PQ Template associated qualification testing. This would result in the in a much better under standing of how to produce a superb combined IQ OQ PQ Protocol at greatly reduced cost.
IQ OQ PQ Template Evolution.
All of our three part combined IQ OQ PQ template and it must be understood that comprehending; How to produce a superb combined IQ OQ PQ Protocol, can be made easy with the correct introduction to a common standard with the layout, introduction and table of contents being precisely and repeatable similar.
While all the test and inspection stages are purposely targeted at the validation online requirements of the specific item under qualification.
The IQ section of the combined IQ OQ PQ template contains all the standard qualification inspections and tests that you would include in a stand-alone Installation Qualification Protocol for the same equipment. The Operational Qualification section of the combined IQ OQ PQ template in a similar manner contains all the standard tests and inspections that are usually included in a stand-alone OQ protocol and the Performance Qualification section follows the same pattern.
All three IQ OQ PQ protocol sections contain spare test script pages; that can easily be edited to suit any specific additional tests that may be required.
So why not ask about validation online time-line savings made in using these three part protocols ? Which are still held as three separate tasks (for compliance purposes); but are published as one document (for cost effectiveness). I.E. One introduction to be edited. One rationale to be edited - Test scripts all written up for you - One document to be circulated for peer review - One document to be approved and printed. Only one document to be managed and maintained through from conception to execution and through change control and on into archive.
The case for the combined IQ OQ PQ template qualification protocol is very compelling.
Consider 10 items requiring validation qualification that means it could be just ten IQ OQ PQ template as opposed to thirty to write, review, edit and approve. Make no mistake; reviewing and approving documents is always the task that is least planned for and subsequently often a serious bottle neck in achieving time line requirements.
Note:-
Most editing is interactive, i.e. entering the basic data; once, (Company name/equipment details, etc.) immediately inserts the entered data throughout the whole qualification document.
Planned Reference between validation documents.
The charts shown below indicates the relationship between the various validation documents and protocol templates. It can be easily recognized that each of these documents and protocols play an essential role in the validation task. All these documents must be considered mandatory in the majority of instances. However there are instances where the Combined IQ OQ PQ template can be used to replace individual protocol documents.
Essential protocols and documents include, the combined IQ OQ PQ and the DQ, along with the essential VMP, VP, URS, VRA
Understanding how to produce a superb combined IQ OQ PQ protocol
Understanding how to produce a superb combined IQ OQ PQ Protocol is really easy when it is prefaced by a 10 page SOP, which enables you to follow the SOP instructions and progressively (page by page) convert this your fully detailed template into a superb Combined IQ OQ PQ protocol.
These protocol templates are targeted at equipment and are suitable for all equipment from the laboratory to the process line. All standard verification's are already included in the protocol format and all test scripts are fully documented and referenced.
Validation Online documents should never be confused with the routine document templates available on the internet. These templates are mainly little more than a list of chapter headings. Where as, Validation Online documents are of a unique interactive design which has been developed over the last ten years. Ten years during which regulatory compliant documentation has been successfully supplied to over sixty countries.
The theme of how to produce a superb combined IQ OQ PQ Protocol was developed by a team of pharmaceutical consultants working across the pharmaceutical, medical device and bio-technical industries. These consultants covered all the validation disciplines and collectively pondered long and hard to come up with a design that would be acceptable to all users and highly cost effective for companies.
Now this new combined IQ OQ PQ Template protocol brings a refreshingly simple and attractive approach for the industry professional, enabling them to raise professional quality validation online protocols in a very efficient and cost effective manner.
IQ OQ PQ Template Standards
The following method of construction must be used in the; how to produce a superb Combined Protocol. The over-all protocol standards are shown in the SOP’s for the different protocols, here we are concerned about the testing element alone. All testing must be detailed and pre-approved by a qualified person to ensure the system under test has been adequately tested. Each test must comprise of;
Main Sub-headings in Test Script.
- A Rationale; giving the reason and or object of the test.
- A Detailed Test Method.
- A Detailed Acceptance Criteria.
- A Test Result (did test result satisfy the acceptance criteria)
General details that must be adhered to.
- The test result must be initiaied (or signed) by the person executing the tests, on completion or at each significant stage.
- Training given for understanding the intricacies of how to produce a superb combined IQ OQ PQ Protocol.
- Each test must be designed to verify an element of the equipment functionality.
- Each test must a have a result that is clear, unambiguous and known.
- The test method must call up for the recording of the test result parameters. (no ticks or tick boxes, no generalities).
- Each test in this combination IQ OQ PQ Template protocol must be witnessed or the results must be reviewed by a competent person.
- The overall test results must be approved by a competent person.
Combined IQ OQ PQ Template
News for Today
FREE VALIDATION RISK ASSESSMENT SOP
Combined IQ-OQ-PQ Equipment. (Issue-8) -- $159.00
This unique validation protocol arrives with you fully detailed with all test scripts, rationales and test methods laid out in accordance with regulatory expectations. The document was specifically designed to be compliant with the all of the FDA, EC and WHO legislation and guidance documentation and over 1700 have been used in submissions to regulatory authorities. How to produce a superb How to produce a superb oombined IQ OQ PQ protocol along with the appropriate interrelated plans and assessment documents will not only validate the item under qualification but it will also produce the thorough audit trail needed to meet all internal or external regulatory reviews and or inspections.
$159.00 Combined IQ-OQ-PQ Computer (Issue-4) -- $159.00
This combination IQ OQ PQ Template has been produced in response to several hundred Validation Online reader suggestions we received in our ‘Suggestions Section’. It has been carefully designed to make it the preferred choice for Process and Laboratory stand alone equipment. It is interactive, easy to use and suitable for all mixes of equipment with and without software.
Combined IQ/OQ/PQ for Spreadsheets. (issue-2)
$159.00
This combination IQ OQ PQ Template has been specifically designed to verify that all aspects of your spreadsheet conform to best practice and that the spreadsheet layout ensures consistent and accurate use and results. The tests and inspections normally authored in separate protocols have been assembled in one protocol which is divided into three sections. This protocol enables you to verify that your developed spreadsheet application is GMP compliant, thus avoiding 483s and warning letters. You can now validate your application with minimal documentation. Equipment Validation Protocol, validation protocol templates,
Combined IQ/OQ/PQ Protocol for Quality Steam (Issue-4)
$185.00
This is a very detailed and comprehensively scripted protocol. All the test scripts required for IQ/OQ/PQ execution are in place ready for editing to represent your installation exactly. Tests scripts are very detailed and all calculations are broken down into simple stages. Illustrated equipment hook up diagrams and sequential instructions further ensure that this testing is easily within the skills of the average technician. All sterilization tasks using steam attract the attention of the regulators. There are often serious problems that inhibit or reduce the sterilizing efficacy of steam that remain obscure to the operator and only become apparent to the end user. The power of steam to sterilize is very closely linked to the characteristics of that steam. Routine steam quality testing must be carried out to ensure that your sterilization processes are never compromised. Annual testing of steam automatically attracts auditor attention. Steam quality must be re-verified when ever any changes or disturbances affect a qualified quality steam system. Equipment Validation Protocol, validation protocol template,
Combined Climate Controlled Zone IQ/OQ/PQ (Issue 10)
$159.00
This combination protocol has been produced in
response to several hundred reader suggestions we received in our -
Suggestions Section -. It has been carefully designed to make it the
preferred choice for Process and Laboratory stand alone equipment. It
is interactive, easy to use and suitable for all mixes of equipment with
and without software.
The IQ section establishes documented verification that key aspects of
the equipment adhere to approved design intentions and that the
recommendations of the manufacturer have been suitably considered. The
OQ section establishes that there is documented verification that the
installed system functions as specified. The PQ section gives
documented verification that the equipment performance in its normal
operating environment is consistently and precisely as specified in the
User Requirement Specifications (URS). validation protocol template.
Software IQ/OQ/PQ
Issue - 3
$159.00
This template has been carefully designed to make it the preferred choice for the qualification of embedded and stand-alone software used in the pharmaceutical, medical devices and bio-technical industries. It is interactive, easy to use and detailed.
The IQ section establishes documented verification that key aspects of the software adhere to approved design intentions and that the recommendations of the regulators have been suitably considered. The OQ section establishes that there is documented verification that the installed system functions as specified and that there is sufficient documentary evidence to demonstrate this. The PQ section gives documented verification that the equipment performance in its normal operating environment is consistently and exactly as specified in the User Requirements Specifications (URS) Equipment Validation Protocol.