Introduction to - How to produce superb User Requirement Specifications
How to produce superb User Requirement Specifications
Understanding How to produce superb User Requirement Specifications must be a major ambition in the early days of any cGMP regulated project. This is the time to streamline your whole validation task. No matter whether the system is purely mechanical, or a mix of electro-mechanical, or solely a software program, the successful compilation and execution of the Installation Qualification (IQ) (for installation), Operational Qualification (OQ) (for functionality) and the Performance / Product Qualification (PQ) (for operability), is dependent on a user requirements specification template that directs others in How to produce winning User Requirement Specifications containing clear, concise and testable requirements.
URS Evolution
 User requirements Specification 1321
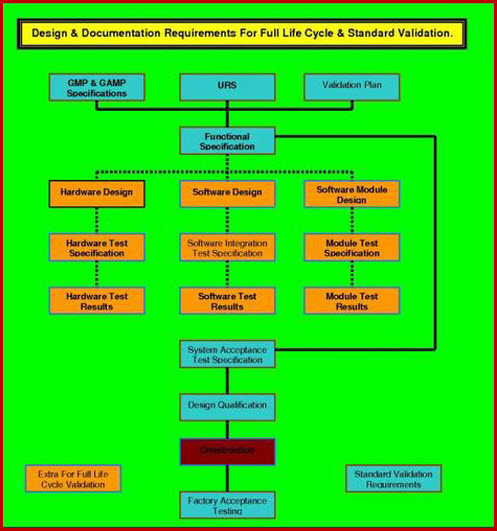
User requirements Specification 1321Understanding how to produce winning User Requirements Specifications templates that can be edited into your own company bespoke protocol and labelled basic URS Level-1 document. The engineers (or vendor) can then commence the preliminary design to establish exactly what functions are required for each of the items specified in the user requirements specification template, the end user requirements has listed. Once this functionality is documented and approved it forms the URS Level-2 document. This is the final level of the URS unless software is used.
If software is to be used, the URS Level-2 document, is passed to the code writers. As the code is written, lines, or groups of lines, of code must be attributed to the individual functions that necessitate their presence. The completion of this task results in the completion of the document.
Developing the URS to this level is unique in most industries, but is, standard practice in strictly regulated industries, as it is a major building block in the creation of quality software. The User Requirements Specification template Level-3 document, contains all the traceability which is deemed mandatory for software assessed to be critical to product quality, in the pharmaceutical regulated industries.
Determining How to produce superb User Requirement Specifications
 User Requirements Specification 3843
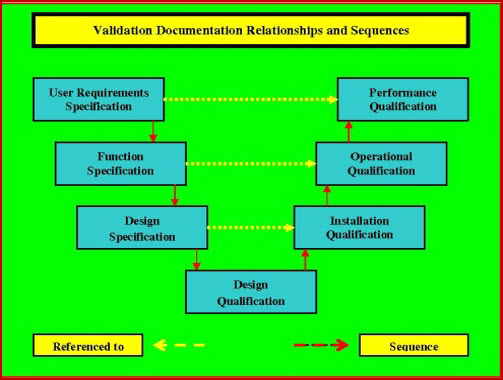
User Requirements Specification 3843Determining, How to produce superb User Requirement Specifications requires careful selection of all tasks in a manner which will verify design; fitness for purpose, has traditionally been a tedious and laborious labor. It involved trawling the VP and URS and cross-referencing to the Functional Specification and the Design Specification and the associated User Requirement Specifications; sometimes, with only limited success. Determining how to produce winning user requirements specification is not only unique, it requires the URS to be an active document up to completion of the Design Qualification (DQ). The DQ will be executed against the three level URS, and verify that the code (if there is any) specified in URS Level-3, will deliver the functionality detailed in User Requirement Specifications template Level-2, which in turn will deliver the operability that the end user requirements specified in URS Level-1.
This document consists of a generic template which uses an attached SOP to allow you to quickly auto-populate the template. It then takes you page by page through the template demonstrating how to produce winning User Requirement Specifications. Just ask about validation time that is saved using this simple and quick to produce quality URS.
User Requirement Specifications Template; Justification
Each and every requirement listed in a User Requirement Specifications template relating to product safety, identity, strength, purity, and quality must be comprehensively identified. Hence, Quality Assurance (QA) must have a significant role in reviewing and approving the final list of requirements, and must be an approver of changes to any user requirement that can affect the above product or process attributes (e.g., cGMP’s).
Given a comprehensive User Requirements Specification Template that has been approved by QA and is under project change management, the Design Qualification (DQ) process then can be reduced to two key objectives:
- Documented verification that the overall design appears to address, by some means, each and every requirement; in the URS, affecting the product and performance of the manufacturing process (or, in the case of unknown product or multi-product manufacturing facility, the required equipment/ system performance capabilities).
- Identification (and documentation) of the critical individual physical components, attributes, and operational features that directly support the concept of How to produce super User Requirement Specifications protocol completely.
User Requirement Specifications; Scope.
 User requirements specification 2217
User requirements specification 2217User Requirements Specification template Scope includes but is not limited to;
- Level-1, full details of end user requirements.
- Level-2, full details of functionality.
- Level-3, software functionality interface.
- A full description of the required system performance.
- Performance criteria, critical parameters and operating range.
- Cleaning and maintenance of user requirements.
- Defining how to produce winning User Requirement Specifications
- Appropriate regulatory How to produce winning User Requirement Specifications template.
- Documentation; How to produce winning User Requirements Specification protocol.
- Training user requirements.
- All none industry standard testing that may be required.
User Requirement Specificationz and the Software Life Cycle.
User Requirement Specifications Template in the Qualification Process.
News for Today
User
Requirements Specification (Issue 8) -- $115.00
The How to produce superb User requirement Specifications How to produce superb User Requirement Specifications diatribe, is probably the most informative and useful data you could read. It sets the standard, and specifies your requirements in a manner that ensures when a system or piece of equipment is selected for cGMP use that all essential support elements; i.e. maintenance, parts, operator & maintenance training, are planned and budgeted for. It uses three levels of User Requirement Specifications Template (URS), URS Level 1, 2 and 3, and is the only URS to guarantee traceability from the URS through to the final OQ and PQ functionality testing. A requirement mandated by cGMP regulations. It can be used on mechanical, electrical and software controlled, monitored or driven systems.
Operational Qualification, SOP & Protocol (Issue 10.) $115.00 10000025
You will find the step by step attached SOP delightfully simple and straightforward to use, as it takes you through the process of customization of your Operational Qualification Protocol template. Following the attached SOP will quickly and smoothly convert your template into an equipment specific Operational Qualification Protocol. The OQ template comes complete with all the standard test scripts, more specialist test scripts can be found listed below. These can easily be pasted into the standard OQ, allowing you to quickly build your own fully detailed and referenced company bespoke Operational Qualification Protocol.
Equipment combined IQ/OQ/PQ Protocol.
$159.00
This combination protocol has been produced in response to several hundred reader suggestions we received in our ‘Suggestions Section’. It has been carefully designed to make it the preferred choice for Process and Laboratory stand alone equipment and associated standard operating procedures. The associated Validation Master Plan template is interactive, easy to use and suitable for all mixes of equipment with and without software. This of course may well be a validation Plan template.
The IQ section establishes documented verification that all validation Online executables are catered for and that key aspects of the equipment adhere to approved design Qualification template and the recommendations of the manufacturer have been suitably considered. The OQ section establishes that there is documented verification that the installed system functions as specified and that there is sufficient documented process validation executables to demonstrate this. The PQ section develops documented evidence that all the requirements specified in your corporate validation 4U manual have been verified as operating consistently and exactly as specified in the User Requirements Specification template.