Current Good Manufacturing Practice.
Current Good Manufacturing Practice Introduction.
Current Good Manufacturing Practice (cGMP) legislation defines the rules and regulation that are mandated by the Food and Drug Agency (FDA) to ensure all production activities are executed by trained operatives following approved documented procedures that utilize only fully validated and calibrated equipment, facilities and utilities. (21 CFR Part 820/210/211/11 Refers.
These FDA regulations use the title - current Good Manufacturing Practice - (cGMP Validation), to describe these guidelines.
Courts may theoretically hold that a drug product is adulterated even if there is no specific regulatory requirement that was violated as long as the process was not performed according to industry standards. By June 2010, the same GLP - cGMP Validation requirements will apply to all manufacturers of dietary supplements. The World Health Organization WHO, version of cGMP Validation is used by pharmaceutical regulators and the pharmaceutical industry in over one hundred countries worldwide, primarily in the developing world. The European Union's cGMP (EU-GMP) enforces similar requirements to WHO cGMP Validation, as does the Food and Drug Administration's version in the US. Similar current Good Manufacturing Practice based validation legislation is used in other countries.
In the United Kingdom, the Medicines Act (1968) covers most aspects of cGMP in what is commonly referred to as "The Orange Guide", which is named so because of the color of its cover; it is officially known as Rules and Guidance for Pharma Manufacturers and Distributors.
Current Good Manufacturing Practice (for medicinal products) is that part of Quality Assurance requirements which ensures that Medicinal products are consistently produced and controlled to the quality standards appropriate to their intended use and as required by the marketing authorization or product specification. Current Good manufacturing Practice is continually concerned with both production and quality control.
Current Good Manufacturing Practice (cGMP), are issued by regulatory authorities throughout the world, country by country and region by region. In general they list minimum standards for the safe manufacture, storage and distribution of medicinal drugs, devices and all associated records. GMP rules and FDA regulations are just as enforceable (in the country of their origin) as is common criminal law. Because pharmaceutical products and their raw materials are manufactured and distributed internationally, collaborations between manufacturing companies industry publishing guidance methodologies such as GAMP-5. in an attempt to introduce commonality in many specifications and standards; this is a continuous and ongoing process.
There are several different sections to Current Good Manufacturing Practice validation Online requirements; as detailed in CFR’s, so it is essential to study those applicable to your processes. I.E. Medical Device, Biotech, Human Food, or Drugs. Manufacturers must adapt a very positive and proactive approach in conforming and enforcing the rules and guidelines throughout their entire manufacturing, inspecting, storing and distribution processes. They are required to implement processes and procedures that comply with the requirements listed in the applicable current Good Manufacturing Practice cGMP, and gain approval from their regulatory authority that they comply with all of these regulations prior to being allowed to release their produce for public or prescribed use. They are subject to continuous monitoring of this regulated state for compliance with the cGMP legislation.
Current Good Manufacturing Practice cGMP.
Current Good Manufacturing Practice cGMP and FDA regulations have been developed to ensure that medicinal pharmaceutical products are consistently produced and controlled to the quality standards appropriate to their intended use. They have been developed and introduced in 1962 in response to the US public’s concern about the safety, efficacy and overall quality of drugs. In the United States the regulations are called cGMP to take into account that the regulations are not static but rather dynamic. They are defined in Title 21 of the U.S. Code of Federal Regulations: current good Manufacturing Practice for drugs in general and 21 CFR 211, for finished pharmaceuticals. In 1996 the FDA proposed a significant revision of the regulation. Any drug marketed in the US must first receive FDA approval, and must be manufactured in accordance with the US Current Good Manufacturing Practice regulations. Because of this, FDA regulations have set an international regulation benchmark for pharmaceutical manufacturing.
In Europe local current Good Manufacturing Practice cGMP, regulations exist in many countries. They are based on the European Union (EU) directive: cGMP for Medicinal Products in the European Community. This EU GMP is necessary to permit free trade in medicinal products between the member countries. Regulations in the EU allow for the marketing of a new drug in the twelve member countries with a single marketing approval.
The EU GMP is intended to establish a minimum manufacturing standard for all member states. The EU directive has been widely harmonized with the Guide to current Good Manufacturing Practice for Pharmaceutical Products as developed by the Pharmaceutical Inspection Convention (PIC).10
CURRENT GOOD MANUFACTURING PRACTICE.
News for Today
Validation Master Plan (Issue 8) -- $115.00
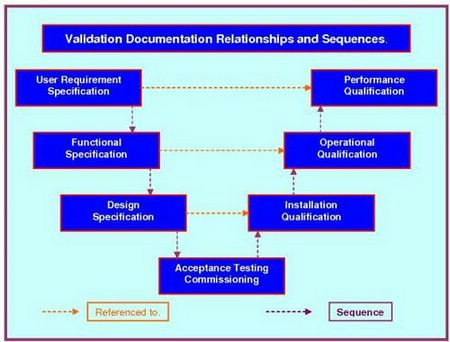
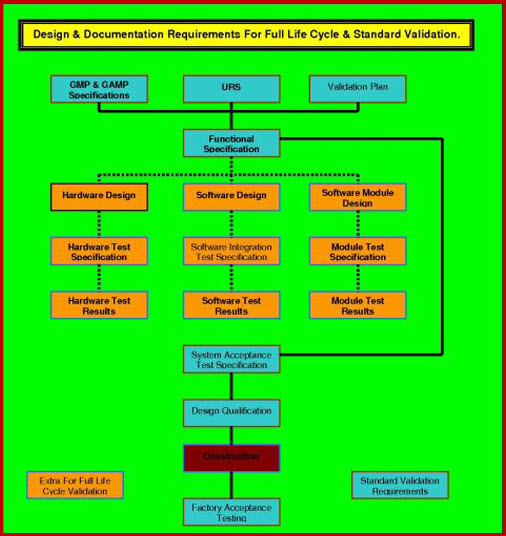
All you need to do is follow the prompts in the attached SOP. They will take you through the completion process; section, by section. At the end of this process your generic document has progressed into a detailed, referenced, bespoke company document. The document follows our three level URS system that ensures functionality traceability from the URS to the various testing protocols, as required by current Good Manufacturing Practice legislation. This document interfaces with our Validation Risk Assessment (VRA), Validation Project Plan (VP), User Requirements Specification (URS), giving a seamless flow from your VMP through the VP - IQ - OQ - PQ, while integrating flawlessly with the URS - DQ - VRA.
Validation Plan (Issue 10) -- $93.00
The Validation Plan (VP), is the starting point for any validation task, & singly the most important validation document. It improves validation efficiency greatly by forcing all concerned to document, review, and discuss, the requirements contained in the "Good Manufacturing Practice" section (cGMP). While in the past validation was more focused on functions of procedures, recently the focus has progressed into infrastructure, networked systems and on security, authenticity and integrity of data acquired and evaluated by systems.
Validation Risk Assessment (Issue 11) $125.00
This is a robust and simple to execute document, one that will lead you through the process and deliver a result that can be used as the foundation for your validation Online activities. The VRA now includes the assessment table for categorizing and documenting the new 21 CFR Part 11 guidance ruling on what predicate data must be stored in a current good manufacturing practice Part 11 compliant system, along with the new broadsheet to establish your new database of part 11 records. (now mandatory).