How to write a superb Operational Qualification
Operational Qualification Template (Rationale).
We are often asked how to write a superb operational qualification protocol and we always advise - simple purchase our template and follow the inbuilt SOP and edit into your own bespoke protocol. You will find you have a very useful and FDA compliant document to work with,
Now that your Operational Qualification template has been edited into into your own cGMP compliant, bespoke & impressive Protocol the Operational Qualification template (OQ) protocol can be circulated for review and approval. This is a really easy and straight forward document to use. In the preparation of Operational validation protocols, it is important to allow a certain degree of flexibility. This must be debated and justified in the Validation Plan (VP). Should the OQ remain untouchable, until the Installation-Qualification (IQ) is completed and signed off? There are many instances where this is not only undesirable, but senseless and deleterious to project progress and costs. Should every function in a system or piece of equipment be qualified? If so, one must ask about validation progress and costings. It makes little sense to fail something for not reaching a parameter that you are not going to use.
The How to write a superb operational qualification template must include a review of the Standard Operating Practice (SOP's) for start-up, operational, maintenance, safety, and cleaning / sanitization. The modular process has been followed in constructing this Operational Qualification template, in as much that where tests / inspections are standard for systems and or equipment, they are built into the basic protocol. Where they are not they are available as additional test protocols.
Functional and software tests are authored in this stand alone test scrip protocol format. When the OQ is being raised they are pasted in. The document format will paginate them, and automatically add them to the ‘Table of Contents’. They are then part of the Operational qualification template. These Test Scripts can be held as Method Statements or SOP's. This allows the generation of a standard OQ that covers all the many items the regulators are looking for, with the facility to have integrated into it, the equipment specific testing tasks. It also means that these stand alone test scripts are available for tasks other than validation, i.e. when system re-testing is required.
It also saves unnecessary testing and re-testing. For example, when a room pressure regime has been commissioned by another party and the completed report is in date and available, is it practical and legal to use it? There is no reason why not, providing it has been authored, and executed using the same documentation practices and procedures as are used in the routine production and execution of validation protocols. The OQ test scrip will be of standard format, the contractors tests (containing the raw data) will be reviewed and data extracted from it.
This data will be used to verify that the Operational Qualification template test script acceptance criteria has or has not Operational Qualification template Rationale. been satisfied. The raw data from the contractor now forms part of the OQ, and must be appended to it permanently, along with copy calibration certificates for all the test equipment used in obtaining the raw data.
Where you use your own indigenous testing methods contained in an SOP or a Method Statement (or whatever you decide to call them), they must be used in a manner similar to the manner the contractors raw data was used.
Understanding How to write a superb operational qualification template.
You will find the step by step attached SOP delightfully simple and straightforward to use, as it takes you through the process of customization of your Operational Qualification template. Following the attached SOP will quickly and smoothly convert your template into an equipment specific Operational Qualification Protocol. The OQ template comes complete with all the standard test scripts, more specialist test scripts can be found listed below. These can easily be pasted into the standard OQ, allowing you to quickly build your own fully detailed and referenced company bespoke Operational qualification. Template for VMP.
The scope of the OQ testing/inspections must include but is not limited to:
- Verification that all operational qualification template identified loops are calibrated.
- Insert a brief description of what part of the validated product process.
- Insert a brief description of the operational function.
- An integrated loop test verification.
- Testing of alarms.
- Testing of interlocks and permissive conditions.
- Testing of database or data storage integrity.
- Testing of report generation and event chronicle.
- Verification of the functionality of the equipment.
- Challenge of software, where required;
- Review of system functionality to verify compliance with 21 CFR Part 11.
- If system must be 21 CFR Part 11 compliant, verification;
- Testing of security levels to prevent;
- Testing to verify and document 'Power loss Recovery'.
- Verifying how to write a superb operation qualification Testing of all functionalities;
- Testing for Electromagnetic interference and compatibility.
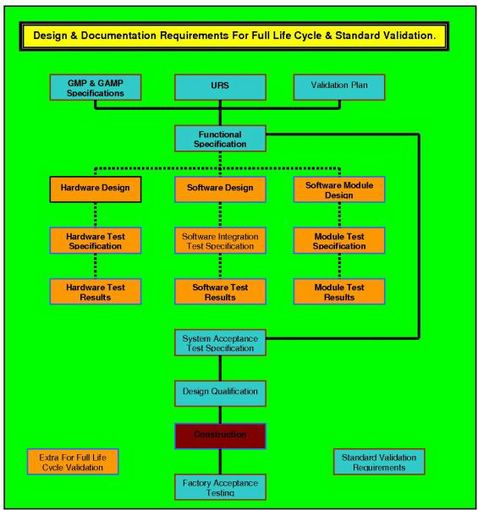
Full Life Cycle Documentation.
News for Today
Operational Qualification Template (Issue 10.) --$115.00
You will find the step by step attached SOP delightfully simple and straightforward to use, as it takes you through the process of customization of your Operational Qualification Template. Following the attached SOP will quickly and smoothly convert your template into an equipment specific Operational Qualification Template. The OQ template comes complete with all the standard test scripts, more specialist test scripts can be found listed below. These can easily be pasted into the standard OQ, allowing you to quickly build your own fully detailed and referenced company bespoke Operational Qualification Template for VMP.
Validation Plan (Issue 8) -- $93.00
This document follows our well-developed method of using a generic document and allowing the customer to apply an attached detailed SOP to it, turning the generic document quickly into a first class company bespoke document. This VP details and integrates all validation activities and procedures required for a small to medium sized project, involving production / facility / utility equipment using electronic controls or monitoring.
User Requirements Specification (Issue 8.) -- $115.00
The document that sets the standard and specifies your requirements in a manner that ensures when a system or piece of equipment is selected, it will deliver the functions you want; i.e. it will have maintenance standards and methodologies; it will have calibration records; it will have all the documents and records to enable successful validation to be completed. This document was designed to be used as a live document up until the DQ is completed and approved. It uses three levels of URS, URS Level 1, 2 and 3, and is the only URS to guarantee traceability from the URS through to the final PQ and Operational qualification template functionality testing. This is a mandatory requirement for Full Life Cycle Validation (FLCV) of computer systems that are the subject of predicate rules. It can be used on mechanical, electrical and software controlled, monitored or managed systems.
Design Qualification (Issue 5.) -- $115.00
The Standard Operating Procedure attached to this generic design qualification protocol, will chapter by chapter, take you through the task of raising a fully detailed document. The main body is split into fourteen tables, each one probing the design requirements and standards for the individual requirement. Safety and security along with user operability are very detailed. The document will lead you through all these design aspects allowing you to delete some you feel are not important to your equipment. It is an easy document to use and will ensure that you’re DQ’s are relevant, up to date and easy to execute. Practically all the requirements are in table form. Allowing fast and clearly presented results to be obtained.
Installation Qualification (Issue 9.) -- $115.00
The SOP used to generate this IQ, takes you through the process line by line, chapter by chapter. It really is unique to find a SOP document so easy to use, all the work is done for you. All the documents are detailed, all the drawings listed and all the checks and tests detailed. The final product is a professional and comprehensive Installation Qualification Protocol: One that you can produce in less than 60 minutes. Yes, think about it, we all know how long producing IQ documents has taken in the past. There is now no reason for not being able to produce 4 to 8, IQ protocols per 8 hour day.