Producing a winning Operational Qualification protocol.
Producing a Winning Operational Qualification Protocol.
Producing a winning Operational Qualification protocol is greatly simplified by the basic design of the protocol template which includes an integral interactive and intuitive lead through SOP. Making this a a really easy and straight forward document to use. In the preparation of the Operational Qualification validation protocols, it is important to allow a degree of flexibility. This must and should be specified in the
Validation Plan (VP). Should the Operational Qualification remain untouchable until the Installation-Qualification (IQ) is completed
and signed off? There are many instances where this is not just undesirable but senseless and deleterious to project progress
and costs. Should every function in a system or piece of equipment be qualified? It makes little sense to fail something for not reaching a parameter that you are not going to use. The Operational Qualification must include a review of the Standard Operating Procedure (SOP's) for start-up, operation, maintenance, safety, and cleaning / sanitization as applicable, must they be in fully approved format? These flexibilities must be built into the qualification process. However there is an abundance of rules and guidelines that are not flexible, and must be rigorously adhered to. The modular process has been followed in constructing this Operational Qualification, in as much that where tests / inspections are standard for systems and or equipment, they are built into the basic protocol. Where they are not they are available as test protocols. Functional and software tests are authored in this stand alone test scrip protocol format. When the OQ is being raised they are pasted in. The document format will paginate them, and automatically add them to the ‘Table of Contents’. They are
then part of the OQ. These Test Scripts can be held as Method Statements or SOP's. This allows the generation of a standard Operational Qualification that covers all the many
items the regulators are looking for, with the facility to have integrated into it, the equipment specific testing tasks. It also means that these stand alone test scripts are available for tasks other than validation, i.e. when system re-testing is required.
Operational Qualification Definition.
"Execution of the Operational Qualification protocol must produce sufficient data to verify that; all the operation functionalities mandated in the User Requirements Specification, have been fully complied with."
Rationalizing of Inspection and Test Occurrences.
It also saves unnecessary testing and re-testing. For example, when a room pressure regime has been commissioned by another party and the completed report is in date and available, is it practical and legal to use it? There is no reason why not, providing it has been authored, and executed using the same documentation practices and procedures as are used in the routine production and execution of validation protocols. The OQ test scrip will be of standard format, the contractors tests (containing the raw data) will be reviewed and data extracted from it. This data will be used to verify that the Operational Qualification test script acceptance criteria has or has not Operation Qualification Rationale.been satisfied. The raw data from the contractor now forms part of the OQ, and must be appended to it permanently, along with copy calibration certificates for all the test equipment used in obtaining the raw data.
Whether you use your own indigenous testing methods contained in an SOP or a Method Statement (or whatever you decide to call them), they must be used in a manner similar to the manner the contractors raw data was used.
PLEASE CLICK HERE AND GO TO OUR STORE.
Operational Qualification Scope.
The scope of the Operational Qualification testing/inspections must include but is not limited to:
- Verification that all loop installations;
- Insert a brief description of what part of the validated product process.
- Insert a brief description of the operation function.
- An integrated loop test verification.
- Testing of alarms.
- Testing of interlocks and permissive conditions.
- Testing of database or data storage integrity.
- Testing of report generation and event chronicle.
- Verification of the functionality of the equipment.
- Challenge of software, used in Producing a Winning Operational Qualification
- Review of system functionality to verify compliance with 21 CFR Part 11.
- If system must be 21 CFR Part 11 compliant, verification;
- Testing of security levels to prevent;
- Testing to verify and document Power
loss Recovery.
- Testing of all interfaces between;
- Testing for
Electromagnetic interference and compatibility.
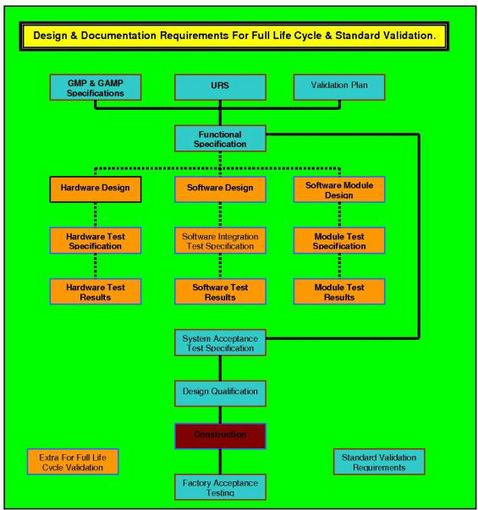
Full Validation Life Cycle Documentation
News for Today
SOP for Spreadsheet Creation. -- $125.00
Why
does something as simple as a spreadsheet figure in so many regulatory
citations? Good question; and at times a difficult one to answer. When
you ask a group of compliance personnel the same question you will be
informed that Excel cannot be validated because it does not seal the
original copy (of the spreadsheet), allows the original to be modified
and has an audit trail that can be disabled. All true, but none of
these problems interfere with your ability to validate that the
spreadsheet is fit for purpose. They only preclude you from using the
spread sheet as a compliant repository for any data that has to be store
in compliance with 21 CFR Part 11.
If the spreadsheet is signed off and dated by the user, their supervisor
and QA, it becomes regulatory acceptable data stored in hard copy, and
Part 11 does not apply.
After numerous requests for this, we have launched our brand new SOP for
Spreadsheet Creation to cover these and other known target points that
the regulators consistently hone into as soon as they find that
spreadsheets are being used. Use this Spreadsheet Creation SOP to ensure
that you create spreadsheets that are validatable. Then use our
spreadsheet validation pack to validate them.
Combined IQ/OQ/PQ Equipment. (Issue-7) -- $159.00
Equipment combined IQ/OQ/PQ Protocol.
This combination protocol has been produced in response to several
hundred reader suggestions we received in our ‘Suggestions Section’. It
has been carefully designed to make it the preferred choice for Process
and Laboratory stand alone equipment. It is interactive, easy to use
and suitable for all mixes of equipment with and without software.
The IQ section establishes documented verification that key aspects of
the equipment adhere to approved design intentions and that the
recommendations of the manufacturer have been suitably considered. The
OQ section establishes that there is documented verification that the
installed system functions as specified and that there is sufficient
documentary evidence to demonstrate this. The PQ section gives
documented verification that the equipment performance in its normal
operating environment is consistently exactly as specified in the URS.
Combined IQ/OQ/PQ for Spreadsheets. (issue-2) $159.00
This validation online combination protocol has been specifically designed to verify that all aspects of your spreadsheet conform to best practice and that the spreadsheet layout ensures consistent and accurate use and results. The tests and inspections normally authored in separate protocols have been assembled in one protocol which is divided into three sections. This protocol enables you to verify that your developed spreadsheet application is GMP compliant, thus avoiding 483s and warning letters. You can now validate your application with minimal documentation. Equipment Validation Protocol, validation online protocol template.