How to write a superb Installation Qualification.

Installation Qualification (IQ) Rationale.
Understanding how to write a superb Installation Qualification protocol that is compliant with current regulations and capable of verifying that the equipment, and its ancillary systems or sub-systems have been installed in accordance with installation validation drawings and or specifications. It further details a list of all the cGMP requirements that are applicable to this particular installation qualification. These requirements must all be satisfied before the IQ can be completed and the qualification process is allowed to progress to the execution of the IQ
In the past equipment suppliers have often been delinquent in supplying the right scope and quality of support documentation for their products. As a consequence end user companies have not always had sufficient information (especially in the form of engineering drawings and specifications) about the equipment they have purchased, to develop and put into place the quality of documentation that regulatory requirements demand. For correct and trouble free installation qualification it is really essential that the scope and quality of the proposed equipment documentation; is specified at the IQ procurement stages.
Installation Qualification (IQ) Introduction
You will find that our How to write a superb installation qualification protocol (IQ) comes with an interactive SOP as an attached prefixed document. As you follow the installation validation protocol requirements as specified in the SOP, you complete the actual IQ protocol. This makes it a really easy and straight forward document to use. The Installation Qualification is normally a stand alone document, however, with careful pre-planning, certain aspects of the IQ activities can be integrated with the Factory Acceptance Testing (FAT), and the equipment Commission Testing.
Conformance with current Good Manufacturing Procedures. (cGMP's) requires, that what ever approach is used, it is fully documented in the individual
Validation (Master) Plan
(VMP or VP).
The IQ should not start with the Factory Acceptance Testing (FAT) or Commissioning tasks, but it should start before these tasks are completed; enabling the validation team to witness and document the final FAT and commissioning testing. The integration of these activities greatly reduces the costly and time consuming replication of unnecessary retesting.
There is a grey area of testing / inspection in the transition from the IQ to the Operational
Qualification (OQ) that is open to rationalization, i.e. it has to be done, but it can satisfactorily be included in either the Installation Qualification or OQ. On most projects the simpler the IQ is
kept, the quicker it is completed,
reviewed and out of the way, allowing progress to the OQ. It can therefore pay dividends to keep the IQ
as basic as possible. Where possible, the IQ can be kept to the pre-powering up stage. Keeping the
Installation Qualification at
this level allows healthy project progress. There should be no
powering up or utility problems to hinder IQ sign off, and rapid progress through the OQ to the Performance
Qualification (P1Q) and where applicable the Process Qualification (P2Q),
becomes feasible. It can be reasoned that as soon as you power up, you enter the equipment operation stage, where verification of the User
Requirements Specification (URS) and 21
CFR Part 11 verification testing are undertaken.
For this reason we have modularized the; How to write a superb Installation Qualification template, building in the tests and inspections hat are not in this grey area, and building the one's that are, as modules that can be added to either the IQ or the OQ. This has not been done just because it might work, this has been advocated by us for some time. We have experience major delays to projects, caused purely by waiting for the client to complete document reviews and approvals. See Comment 9 in cGMP-FDA-483.
Conformance with current Good Manufacturing Procedures. (cGMP's) requires, that what ever approach is used, it is fully documented in the individual Validation (Master) Plan (VMP or VP). The IQ should not start with the Factory Acceptance Testing (FAT) or Commissioning tasks, but it should start before these tasks are completed; enabling the validation team to witness and document the final FAT and commissioning testing. The integration of how to write a superb installation Qualification protocol greatly reduces the costly and time consuming replication of unnecessary retesting.
Understanding How to write a superb Installation Qualification protocol
The scope of the Installation Qualification testing/inspections will cover the under listed subjects, but is not restricted to them alone.
- Verification that all components parts ~.
- Verify installation is as specified ~.
- Insert a brief description of what part of the product
process ~~..
- Insert a brief description of the
operational function ~~. .
- Verify supply voltages conform with ~~.
- Verify that
electrical installation qualification complies with ~~.
- Verify that all alarms and visual displays are ~~.
- Verify that where used, the correct versions of ~~.
- Confirm that the issue level of software ~~.
- Identify and verify that serial
numbers of all ~~.
- Review all
calibration certificates for ~~.
- Verify where used, there is signals continuity~~.
- Verify signal continuity between ~~.
- Verify that the ambient conditions are ~~.
- Verify that the documentation provided by the ~~ .
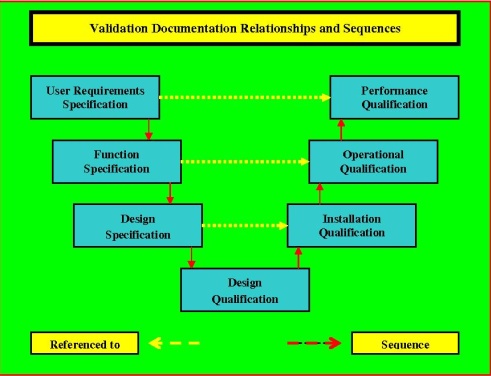
Relationships Within Validation Documentation
News for Today
Installation Qualification
Template
S39.00
The Installation Qualification (IQ) execution; verifies that the equipment, and its ancillary systems or sub-systems have been installed in accordance with installation drawings and or specifications. It further details a list of all the cGMP requirements that are applicable to this particular equipment qualification. These requirements must all be satisfied before the IQ can be completed and the Equipment qualification process is allowed to progress to the execution of the (OQ).
User Requirements Specification Template
$115.00
The User Requirements Specification template is the document that sets the standard, and specifies your requirements in a manner that ensures when a system or piece of equipment is selected for cGMP use that all essential support elements; i.e. maintenance, parts, operator & maintenance training, are planned and budgeted for. It uses three levels of User Requirements Specification Template (URS), URS Level 1, 2 and 3, and is the only URS to guarantee traceability from the URS through to the final OQ and PQ functionality testing. A requirement mandated by cGMP regulations. It can be used on mechanical, electrical and software controlled, monitored or driven systems.
Corporate Validation Manual $1,160.00
This definitive 1000 + page (including all attachments) Corporate Validation Manual arrives with you in USB memory stick format, this enables you at any time to download protocol or test-scrips documents and quickly edit them into company bespoke documents. In fact there are over $3,500.00 worth of superb documents, that form attachments to the DVM manual, which can be instantly copied. Once copied, the unique document interactive editing, allows you to produce high quality bespoke company documents; Such as the Validation Plan Template or the equally ubiquitous User requirements Specification template Design Qualification (DQ); in a few hours. The cost of the Definitive Validation Manual, will be recouped in the first few weeks of use. It will then go on to show a massive return on your original investment.