VALIDATION EXECUTABLES
Defining the Essentials
Validation in the Pharmaceutical, Medical Device and Bio-technical industries is defined as:
The planned verification of a group of inter-related specifications by-way-of tangible evidence.
All test methodologies and equipment used in the validation process must be validated and or calibrated (as specified). All test standards must be traceable to national standards.
Validation Executables Explained.
The definitive list of validation executables can always be found in a professionally prepared suite of validation documents. The format and content used in Professional documents are usually copies of used documents that have been, used and re-edited several times. Which means they have been in front of the regulators before and used satisfactorily. The dread of finding; late in the project execution, some aspect of the system that has not been catered for; or even, things that can't be catered for at all, is just too horrific to contemplate: But not unknown. So we author and register the following documents:
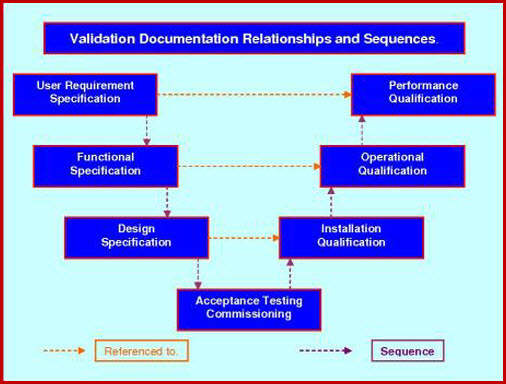
Conventional: VP - URS - VRA - DQ - IQ - OQ - PQ
Since these documents sequentially cascade from the VP through URS, VRA, DQ, IQ, OQ, PQ and finish with the P2Q (when both a performance and a process validation are required). This progression is of the utmost importance since the documents are inter-referenced and are required to be executed sequentially. This is why it is important that the documents are designed and written as a package; where, the correct cross document sequential and chronological requirements can be authored in.
Advanced: VrrP 4Q
In the new Validation risk & requirements Plan (VrrP) document, the contents of the original VP, VRA & URS have been brought together and edited into a more user-friendly format. The new format is much easier to work with and simpler to collate and edit. This document now compliments our equally new 4Q Protocol, which has evolved from the editing and collation of the old format DQ/IQ/OQ/PQ documents; without the cGMP integrity of each individual protocol being compromised. This is an essential step forward for companies seeking to reduce validation costs without infringing regulatory standards.
Validation Executables Responsibilities.

Numerous validation inquiries led us into collating various validation executables to form validation executables suites containing all the required protocols; along with the supporting documents, such as the VP and the VRA. This simplifies the user selection task. The packages have been arranged for three levels of validation executables for process, production, facility and utility equipment and separate bespoke packages for Software, HVAC, Networks and other stand-alone systems.
Validation Executables Packages.
The complete chain of regulatory required documentation required to enable satisfactory for the validation executables of a computer / DCS / PLC / ERP, system. This Validation executables Package contains one of each of these documents VP - URS - DQ - VRA - IQ - OQ - PQ.
Level One.
You want to validate a relatively minor, new or replacement piece of equipment that is going to be used within a facility that is fully validated and subject to change control.
In this case you require :- IQ, OQ, PQ.
Level Two.
You want to validate a process line that has been subjected to major modification, but is validated and is continuing to use the same processes to produce the same product.
In this case you require: VP, URS, IQ, OQ, PQ.
Level Three.
You have a new facility and you want to validate it. In this case you require:- VMP, The global master plan for the whole facility.
cVMP, The master plan for all computer systems. VP, which will define validation executables boundaries, methodologies and responsibilities.
URS, DQ, VRA, IQ, OQ, PQ. For each piece of equipment.
PLEASE CLICK HERE AND GO TO OUR VALIDATION EXECUTABLES.
Document Relationships.
The
use of a document packages ensures a multitude of regulatory requirements are
catered for and possibly a similar multitude of pitfalls, blunders and omissions
are anticipated and negated.
VMP & VP Correct Use.
News for Today
User Requirements Specification Template
$115.00
The User Requirements Specification template is the document that sets the standard, and specifies your requirements in a manner that ensures when a system or piece of equipment is selected for cGMP use that all essential support elements; i.e. maintenance, parts, operator & maintenance training, are planned and budgeted for. It uses three levels of User Requirements Specification Template (URS), URS Level 1, 2 and 3, and is the only URS to guarantee traceability from the URS through to the final OQ and PQ functionality testing. A requirement mandated by cGMP regulations. It can be used on mechanical, electrical and software controlled, monitored or driven systems.
Corporate Validation Manual $1,160.00
This definitive 1000 + page (including all attachments) Corporate Validation Manual arrives with you in USB memory stick format, this enables you at any time to download protocol or test-scrips documents and quickly edit them into company bespoke documents. In fact there are over $3,500.00 worth of superb documents, that form attachments to the DVM manual, which can be instantly copied. Once copied, the unique document interactive editing, allows you to produce high quality bespoke company documents; Such as the Validation Plan Template or the equally ubiquitous User requirements Specification template Design Qualification (DQ); in a few hours. The cost of the Definitive Validation Manual, will be recouped in the first few weeks of use. It will then go on to show a massive return on your original investment.