How to write a superb Design Qualification protocol
How to Write a Superb Design Qualification
Contemplating how to write a superb Design Qualification protocol always raises cause for concern. There are always those who doubt the experience of the inhouse staff and those who are full of confidence in their abilities. All requests for quotations for equipment destined to be used in the production of cGMP controlled product are subjected to the rigors of Design Qualification analysis. In a similar manner; any “off-the-shelf” items being considered, must be subjected to a similar analysis to verify that their existing design specification is equal to; or better than, the required design specification.
Understanding how to write a super Design Qualification protocol.
A Design Qualification protocol protocol is used at the stage where a design that has been developed from the, VMP / URS /GAMP 5 / cGMP / and other Health and Safety Guidelines, is reviewed and documented by competent persons to ensure that the designed equipment, if built, will satisfy all the detailed specified requirements, as contained in the VP and URS.
I have written before in these documents that certain things are critical, well here we are again. You have taken great trouble to write, and have approved, a URS and a VP, now a vendor (or could be in house) has come forward and presented a design that they have prepared, and they state it will satisfy your requirements. This is where the majority of project validation problems evolve from; not immediately apparent, but materializing later in the project time line.
The Design Qualification template is the major document that is going to confirm that the design will work. It must be authored and executed by suitably qualified persons who are knowledgeable enough to challenge the proposed design and its performance. If you have no such persons on your staff you must contract them in, or contract the authoring of the Design Validation protocol out.
When I arrive on site to manage a project, my very first task is always to get to grip with the design, get all the drawings and review them. I do this because thirty years of experience has made me very aware, that I need to know the design is good. So often; this is not the case, and very often there are glaring abnormalities. When these are highlighted with the client and their vendors, only the vendors are smiling. The client had accepted the design and the vendor had quoted for that design, any changes will be extra to the quoted price. Sometimes this has run into seven figures.
A PROPER DESIGN QUALIFICATION IS ESSENTIAL TO YOUR HEALTH.
A Design Qualification template can also be used where a company has prepared a User Requirements Specification (URS) for a piece of equipment and is searching for a manufacturer, but is offered equipment "Off-The-Shelf". A Design Qualification template can be used to verify whether the "off-the-shelf" item will fully deliver the functionality detailed in the URS, and conform to the requirements specified in the VMP / cGMP while complying with all applicable Health and Safety Notices.
PLEASE CLICK HERE TO GO TO SHOP.
Design Qualification Template Scope.
To understand how to write a superb design Qualification can also used where a company has prepared a User Requirements Specification (URS) for a piece of equipment and is searching for a manufacturer, but is offered equipment "Off-The-Shelf". A Design Qualification protocol can be used to verify whether the "off-the-shelf" item will fully deliver the functionality detailed in the URS, and conform to the requirements specified in the VMP / cGMP while complying with all applicable Health and Safety Notices.
Initially the protocol writers must be assessed to ensure that are knowledgeable in the basics of how to write a superb design qualification protocol.
Verification that the design is cGMP compliant, and where software is used; conforms to the life cycle model requested in the VP and detailed in GAMP 5.
Verification that the design qualification template complies with the VMP.
Verification that all the required support documentation is specified.
Verification that the system will be calibratable
Verification that the design will achieve the URS requirements.
Verification that the utility services required are available and validated.
Verification that all the required support documentation is specified.
Verification that the system will be maintainable.
Verification of operation staff training requirements.
Verification that the system will operate in a manner safe to both product and staff.
Verification that the design qualification template conforms to all applicable national standards and guidelines.
Contemplating how to write a superb Design Qualification protocol always raises cause for concern. There are always those who doubt the experience of the inhouse staff and those who are full of confidence in their abilities. All requests for quotations for equipment destined to be used in the production of cGMP controlled product are subjected to the rigors of Design Qualification analysis. In a similar manner; any “off-the-shelf” items being considered, must be subjected to a similar analysis to verify that their existing design specification is equal to; or better than, the required design specification.
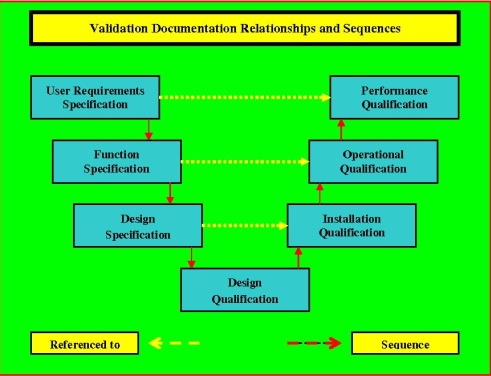
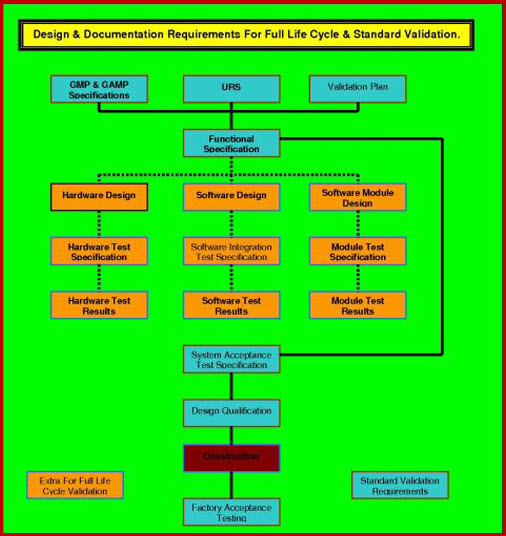
DQ in the Validation Process.
News for Today
User Requirements Specification Template
$115.00
The User Requirements Specification template is the document that sets the standard, and specifies your requirements in a manner that ensures when a system or piece of equipment is selected for cGMP use that all essential support elements; i.e. maintenance, parts, operator & maintenance training, are planned and budgeted for. It uses three levels of User Requirements Specification Template (URS), URS Level 1, 2 and 3, and is the only URS to guarantee traceability from the URS through to the final OQ and PQ functionality testing. A requirement mandated by cGMP regulations. It can be used on mechanical, electrical and software controlled, monitored or driven systems.
Corporate Validation Manual $1,160.00
This definitive 1000 + page (including all attachments) Corporate Validation Manual arrives with you in USB memory stick format, this enables you at any time to download protocol or test-scrips documents and quickly edit them into company bespoke documents. In fact there are over $3,500.00 worth of superb documents, that form attachments to the DVM manual, which can be instantly copied. Once copied, the unique document interactive editing, allows you to produce high quality bespoke company documents; Such as the Validation Plan Template or the equally ubiquitous User requirements Specification template Design Qualification (DQ); in a few hours. The cost of the Definitive Validation Manual, will be recouped in the first few weeks of use. It will then go on to show a massive return on your original investment.