Edit Software Validation template into comprehensive Protocol.
Software Validation is mandatory for all software used in the pharmaceutical process industry. To ease this burden there is a concession that we can investigate the role of the software and where it is minor i,e, when a software fault would be obvious and stop the process; we can validate the functionality. However; where a fault could be obscure or even hidden; then full life cycle validation is mandatory.
To scope and define an adequate software validation template procedure the URS has to be detailed sufficiently for various assessments to be made. The main assessment that concerns us with qualification documentation is the risk assessment. This assessment is only concerned with ensuring that the degree of validation that is proposed; is compliant with the regulatory requirements.
So at this early stage it is required to execute a Validation Online; Risk Assessment protocol against the end user's requirements. This step is purely to ensure that the more obscure pieces of ancillary equipment and support services are fully understood and their requirement investigated, priced and included in the final issue of the URS; which will be sent out with the Request to Tender. This is an essential stage if the URS is to accurately define what depth and scope of software qualification is appropriate for the software verification that the software will deliver all the requirement detailed in the URS.
It is an EU and FDA mandatory requirement that certain aspects of this assessment are documented and held as a regulatory required record. These are;
Does the software validation template online require Full Life Cycle validation ?Does the use of the software verification produce records that must comply with 21 CFR Part 11 ?
If the data requires to be Part 11 compliant, how is that to be being achieved?
The answers to these questions are required to enable the Validation Plan (VP) to provide sufficient information to the protocol writers, to enable them to define the correct content for the Installation Qualification (IQ). Operational Qualification (OQ) and the Performance Qualification (PQ) protocols.
Full Life Cycle Validation.

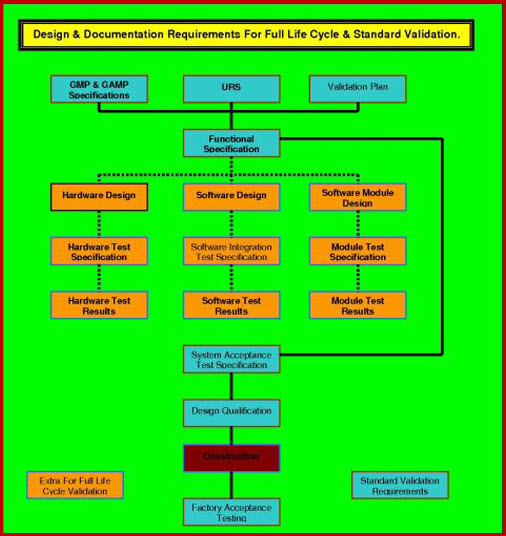
The outcome of the VRA can drive a split in validation requirements documentation scope, if the VRA categorizes the software validation template as requiring Full Life Cycle
Validation FLCV, then a considerable amount of the
software validation template effort is put into establishing how the software was designed
and developed, in order to establish that its basic concept and development can
be considered robust, sound and in accordance with best
practices EU, WHO and FDA legislators.
The original development plans, code reviews, methods reviews and testing plans must be available to enable this Validation Online, software qualification documentation to be executed successfully. Once this proof of quality build is
establish, validation then follows a more convention path in code / procedural /
security verification / functional inspections and verification.
Validation Online software validation template that is not classified as requiring FLCV treatment, does not require this depth of research into quality-build-history and is validated mainly by the more convention path in code / procedural / security verification / functional inspections and verification's.
SOP for Software Validation Template.
The SOP for Software Qualification procedures continues to be an extremely popular document. This document leads you through the validation process, from the URS to the final P2Q.
SOP for Software Validation Template (Issue-1)
Software Validation Template and Testing.
Dynamic Testing.
Validation Online; dynamic testing verifies the execution flow of software, including decision paths, inputs, and outputs. Dynamic testing involves creating test cases, test vectors and oracles, and executing the software validation template against these tests. The results are then compared with expected or known correct behavior of the software. Because the number of execution paths and conditions increases exponentially with the number of lines of code. Testing for all possible traces and conditions for the software verification is impossible.
Static Analysis.
Code
inspections and testing can reduce coding errors; however, experience has shown
that the process needs to be complemented with other methods. One such method is
static analysis
. This somewhat new method largely automates the software validation template online process. The technique
attempts to identify errors in the code, but does not necessarily prove their
absence. Static analysis is used to
identify potential and actual defects in source code.
Abstract Interpretation Verification.
A code verification solution that includes abstract interpretation can be instrumental in assuring software validation template and a good quality process. It is a sound verification process that enables the achievement of high integrity in embedded devices. Regulatory bodies such as the FDA and some segments of industry recognize the value of sound verification principles and are using tools based on these principles.
Establish End Users HL.
These must be based on the functionality; as the end user, will see it. They must cover all aspects of using the program and be sufficiently detailed to enable the development team to clearly and concisely understand what the future product plans are.
Define Functionality Requirements.
This stage develops and expands the software validation template end user’s requirements, and drills down to a sufficient level of detail required for the development team. As each detailed requirement document is completed, it is subjected to peer review and acceptance by a representative from development, quality assurance, architecture, product management, and development management.
Define Design Specification
Based on the
high level requirements (URS level 1
), establish a suitable product architecture taking the end user
requirements and the required functionality requirements into account. This task should be structured in
parallel with defining the detailed requirements.
Product Development.
The Validation Online, source code development team executes the detailed requirements and the technical architecture. This process is best commenced only when both parts of the requirements document have been completed, reviewed and approved.
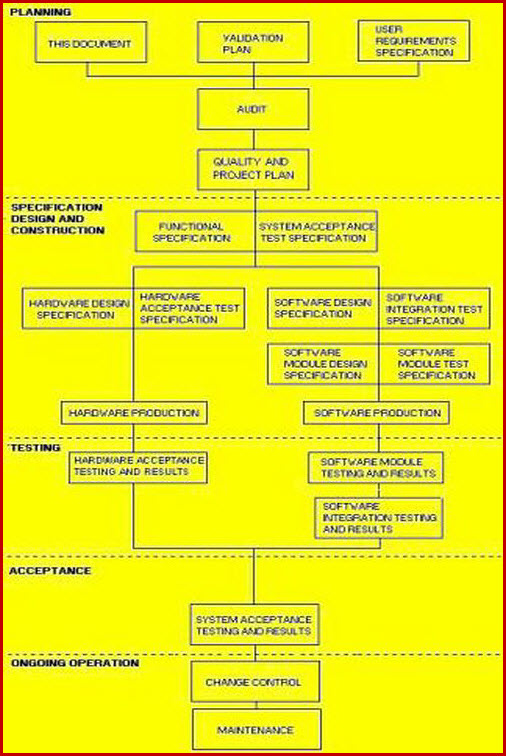
Software Validation Document Flow.
In the diagram below, it can readily be seen that the URS, along with the computer qualification requirements; as given in the VMP, come together to form a document package consisting of the DQ, IQ, OQ and PQ from which the vendor can develop a fully legal and compliant product. Software validation template activities are grouped in two colors (orange and blue). The blue color is the requirement for standard computer qualification.
Quality Assurance.
The QA team must sit in at all stages; from conception to completion and hand over to end user. They are not there to review the software validation template. They are there to ensure that best industry practices are used and that company software policy and procedures are strictly adhered to. They will ensure that the program created by the development team meets the defined requirements and is supplied to the end user complete with all necessary executables and support documentation. They will ensure the source code is installed and used in accordance with company requirements practices & procedures, and all relevant Validation Online cGMP regulations.
SOFTWARE VALIDATION TEMPLATE VERIFICATION.
News for Today
Computer Combined IQ-OQ-PQ (Issue 3) - $ 159.00
This combination protocol has been produced in response to several hundred reader suggestions we received in our 'Suggestions Section'. It has been carefully designed to make it the preferred choice for Process and Laboratory stand alone equipment. It is interactive, easy to use and suitable for all mixes of equipment with and without a software validation requirement.
Computer User Requirements Specification (Issue 5.) - $ 115.00
The document that specifies software validation standards for your computer requirements in a manner that ensures when a system or piece of equipment is selected, it will deliver the functions you want, it will have maintenance standards, it will have calibration records, it will have all the documents and records to enable successful validation to be completed. This document was designed to be used as a live document up until the DQ is completed and approved.
Computer Validation Master Plan (Issue 5.) - $ 115.00
The Computer Validation Master Plan, is the starting point for software validation, and hence the most important validation online document. It greatly improves validation efficiency by forcing all concerned to document, review, and discuss, the proposed methods and allotted responsibilities. It is an expected document with the FDA, and a mandated document with the EU.
CSV Validation Plan (Issue 3.) - $ 89.00
This document follows Validation Online's standard method of using a fully detailed and interactive generic document and enabling to use the attached SOP to quickly convert this generic document into a first class company bespoke document. This Computer System Validation Template, details and integrates all computer validation online activities and procedures required for a small to medium sized project, involving production / facility / utility equipment using electronic controls or monitoring.
Package for Computer Validation Level-1. (Issue 1) - $ 675.00
This software validation package is suitable for all major computer validation projects and contains the underlisted interactive documents. VMP, VP, URS, VRA, DQ, IQ, OQ and PQ.
Package Computer Validation Level-2. (Issue 3) - $ 610.00
The complete chain of regulatory required documentation for the software validation template of a computer system; minus the VMP. This computer Validation Package contains one of each of these documents: VP, URS, DQ, VRA, IQ, OQ, PQ.
Computer Vendor Audit (Issue 3.) - $ 105.00
This software validation vendor Audit document should be customized using the built in tools. The document can then be targeted to reflect your project priorities. The fifteen chapters all contain 10 questions, the total scored is then weighted to reflect your priorities. By assessing the importance of each of the chapter subjects in your project, the weighting is altered taking points from one and adding to others. This enables your assessment to be expressed simple and clearly as a percentage, allowing clear unambiguous comparisons to be presented for competing companies.
CSV Installation Qualification (Issue 6.) - $ 115.00
Computer Installation Qualification (CIQ) is an important step in the overall software validation and qualification process for software and computer validation systems. Our protocol leads you through the detailed requirements.
Operational Qualification (Issue 6.) - $ 115.00
Operational Qualification (OQ) is an important step in the overall software validation and qualification process for software and computer systems. Our protocol leads you through the detailed requirements, progressively and simply.
Computer Performance Qualification (Issue 7.) $ 87.00
The Computer Performance Qualification is the culmination of the computer validation process. The protocol is used in conjunction with the system operating SOP, to verify that the system process is consistent and correct. The results of the testing must be recorder and reviewed with a view to ensuring that any deviations (within permitted tolerances) that exist are random and not a trend that will lead to out of specification operation during production use.