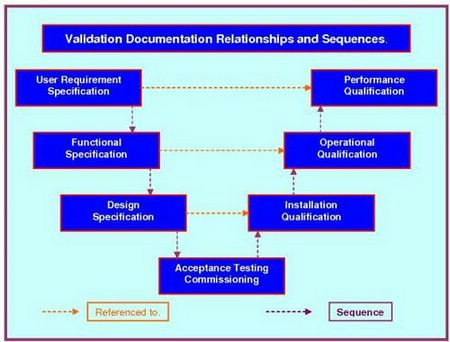
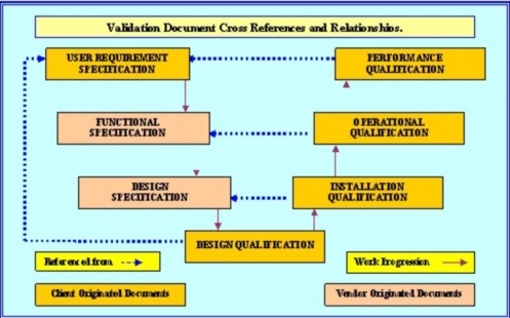
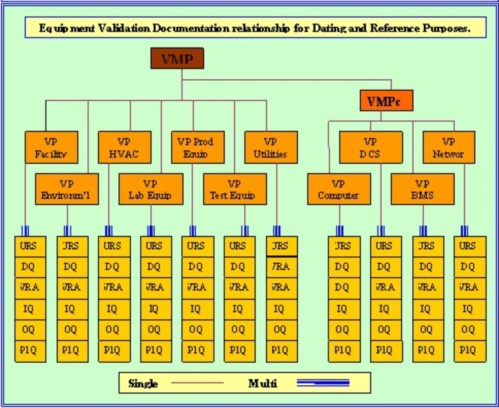
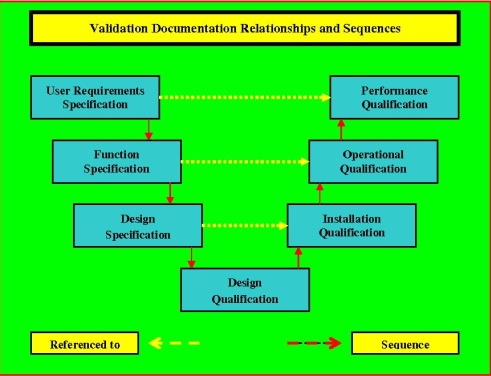
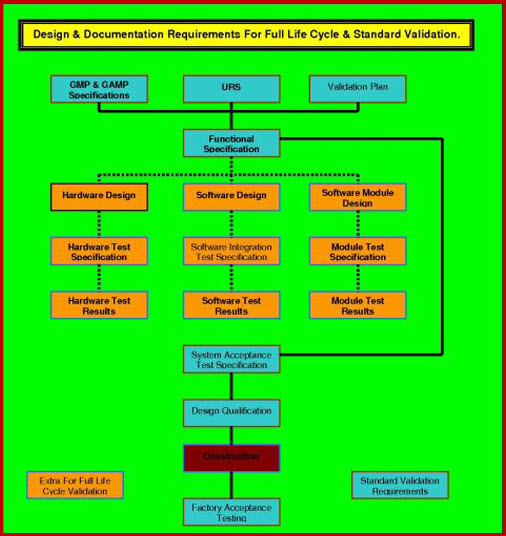
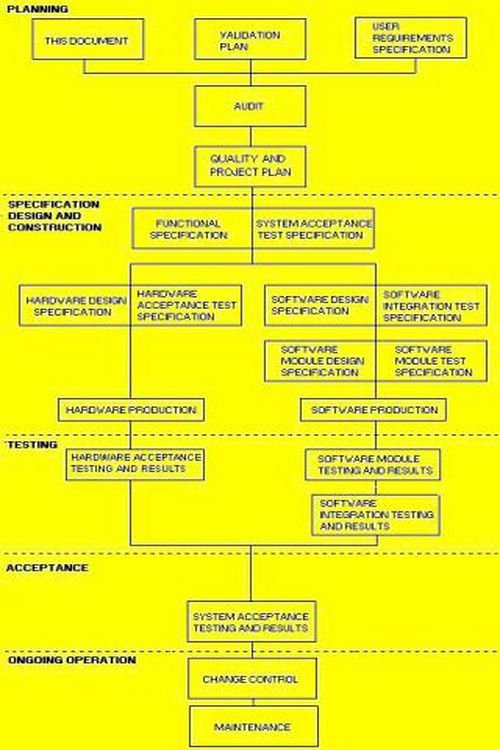
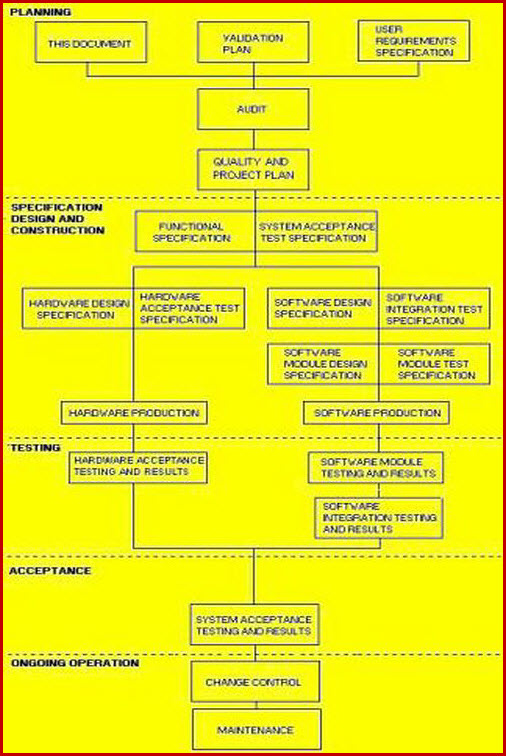
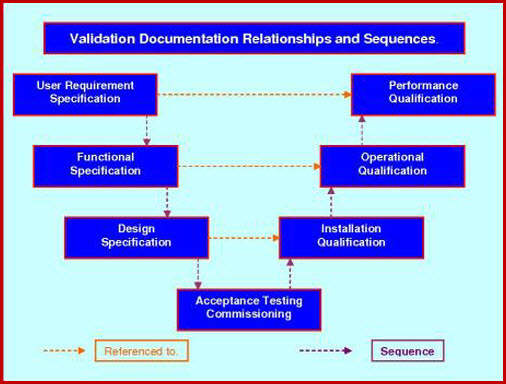
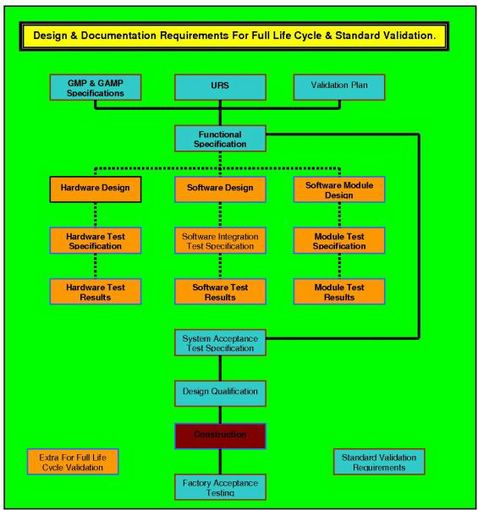
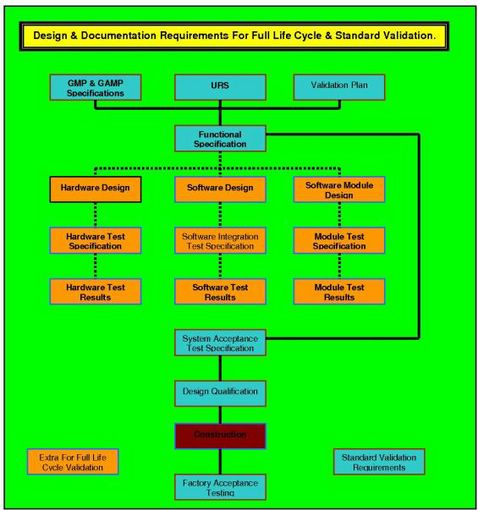
How to write a superb validation Qualification
Validation Online Compliant Templates.
Any auditor: Regulatory or Routine has the right to review your Risk Based Practice and history.
This makes the "Validation Risk Assessment" a Super Critical Document.
Oct 13, 2024
Online Validation
Online Validation guarantees has guaranteed for the last 15 years that purchased documents will be "will be fit for purpose".
Oct 08, 2024
21 CFR Part 211 | FDA | MHRA | EU | WHO | GMP |
21 CFR Part 211 defines verification boundaries, mandates validation executables, documents responsibilities, maintains URS traceability & mitigates risk.
Continue reading "21 CFR Part 211 | FDA | MHRA | EU | WHO | GMP | "
Sep 30, 2024
How to compile and execute a 21-CFR-Part-11 Test protocol
How to compile and execute a 21-CFR-Part-11 Test protocol which will ensure all mandated retention records are held in secure controlled access system.
Continue reading "How to compile and execute a 21-CFR-Part-11 Test protocol"
Jan 25, 2024
How to produce superb User Requirement Specifications
How to produce superb User Requirement Specifications is explained in the interactive lead-through SOP integral to this template.
Continue reading "How to produce superb User Requirement Specifications"
Jan 20, 2024
Current Good Manufacturing Practice - Validation Online
Understand Current Good Manufacturing Practice legislation and ensure your; protocols and plans are compliant with it.
Continue reading "Current Good Manufacturing Practice - Validation Online"
Jan 18, 2024
How to create a winning Standard Operating Procedure
How to create a winning Standard Operating Procedure precisely replicate specific methodologies that were developed to produce a controlled product.
Continue reading "How to create a winning Standard Operating Procedure"
Jan 18, 2024
How to write Retrospective Validation Protocols
How to write Retrospective Validation Protocols that will accurately certify current system compliance with URS..
Continue reading "How to write Retrospective Validation Protocols"
Dec 03, 2023
How to produce a superb combined IQ OQ PQ Protocol.
How to produce a superb combined IQ OQ PQ Protocol that is intuitive to edit & use. Comply's with 21 CFR Part 820/210/211/11 Follow integral lead-thro SOP to complete.
Continue reading "How to produce a superb combined IQ OQ PQ Protocol."
Oct 29, 2023
Validation Online Stats | Packages | Protocols | Plans | SOP's | Test
Validation Online Statistics are recorded and compiled by independent administrators.
Continue reading "Validation Online Stats | Packages | Protocols | Plans | SOP's | Test "
Oct 28, 2023
Compliant Interactive Templates for Computer System Validation
Compliant Interactive Templates for Computer System Validation Simply follow integral SOP to fully bespoke into your own company Protocol.
Continue reading "Compliant Interactive Templates for Computer System Validation"
Oct 21, 2023
How to write a superb Design Qualification.
Understanding how to write a superb Design Qualification protocol is fully explained in the integral SOP built in to each template
Continue reading "How to write a superb Design Qualification."
Oct 11, 2023
How to write a superb Computer Qualification Protocol
Understanding how to write a superb Computer Qualification Protocol is fully explained in the integral SOP built in to each of our templates.
Continue reading "How to write a superb Computer Qualification Protocol"
Oct 08, 2023
SOP - How to Write a Superb Warehouse Climatic Control
Understanding how to write a superb Warehouse Climatic Control protocol is fully explained in the integral SOP built in to each of our templates.
Continue reading "SOP - How to Write a Superb Warehouse Climatic Control"
Aug 28, 2023
How to write superb Medical Device qualification protocols
How to write superb Medical Device Qualification protocols is detailed and defined in the integral SOP contained in all our templates.
Continue reading "How to write superb Medical Device qualification protocols"
Jul 29, 2023
How to write a superb Autoclave Validation
Understanding how to write a superb Autoclave Validation protocol is fully explained in the integral SOP built in to to each of our templates.
Continue reading "How to write a superb Autoclave Validation"
Jul 26, 2023
How to write a superb Validation Plan
Understanding how to write a superb Validation Plan is fully explained in the integral SOP built in to each of our templates.
Jul 24, 2023
Pharmaceutical Validation Instant Download Templates.
Learn the simplicity of using Pharmaceutical validation instant download templates.
Continue reading "Pharmaceutical Validation Instant Download Templates."
Jul 15, 2023
Laboratory Validation Package.
It is an absolute essential that your lab equipment is safely validated. and that you have continuous records that can substantiate this claim.
Jul 15, 2023
Pharmaceutical Equipment Validation
Pharmaceutical Equipment Validation made simple; with or without software. Simply follow Interactive completion SOP & edit into cGMP compliant Protocols.
Jul 15, 2023
PLC Qualification Template | Pack | VMP | VP | VRA | DQ | IQ | OQ | PQ
PLC Qualification Template, FDA,EMA & WHO compliant Protocols, Plans, SOP's, Procedural and Assessment documentation. All available for immediate download.
Continue reading "PLC Qualification Template | Pack | VMP | VP | VRA | DQ | IQ | OQ | PQ"
Jul 15, 2023
Edit Software Validation template into comprehensive Protocol.
Learn software validation techniques that automatically produce the tangible evidence that are a prerequisite for success in all software validation projects.
Continue reading "Edit Software Validation template into comprehensive Protocol."
Jul 15, 2023
SOP for cGMP Review
SOP for cGMP Review. Uniquely Interactive and Highly Cost Effective Documents for FDA and GMP requirements in the USA & Associated Markets.
Jul 15, 2023
SOP-GMP | FDA | EU | WHO | cGMP | QbD | FLCV | SOP's | GxP's |
The SOP-GMP legislative, as published in 21 CFR Part 210/211/820, becomes easier to understand & comply with when professionally authored, interactive & unique protocol templates such as those produced Validation Online; are used, i.e. Planned progress – with no omissions.
Continue reading "SOP-GMP | FDA | EU | WHO | cGMP | QbD | FLCV | SOP's | GxP's |"
Jul 15, 2023
system Hardware Validation | FDA | EU | WHO | Packages | Protocols |
System Hardware Validation docs that are ready for final edit and issue. Docs that not only guarantees compliance, but are also a first class methodology for instilling into validation staff the expectations of their regulators.
Continue reading "system Hardware Validation | FDA | EU | WHO | Packages | Protocols | "
Jul 15, 2023
System Process Air | FDA | MHRA | WHO | EU | GxP | GMP's | SOP's
Never validated System Process Air before? Use an industry standard set of validation protocol templates and follow the in-built fully detailed and interactive SOP to guide you; step by step, to on-time and in-budget project completion.
Continue reading "System Process Air | FDA | MHRA | WHO | EU | GxP | GMP's | SOP's"
Jul 15, 2023
Temperature Safe Transit | FDA | EU | WHO | GMP | QbD | GxP | FLCV |
The most cost method of validation of temperature safe transit systems is undoutably through using industry standard interactive validation templates. Templates which have been specifically designed to be interactive and intuative to use.
Continue reading "Temperature Safe Transit | FDA | EU | WHO | GMP | QbD | GxP | FLCV |"
Jul 15, 2023
Validation A
Validation A is our Interactive cGMP compliant, fully detailed template; then follow the integral completion SOP & proudly sign off, your own bespoke protocol.
Jul 15, 2023
Editing Validation Performance Qualification Template into a Protocol.
Edit this Validation Performance Qualification Template, using the integral SOP to produce your own impressive company bespoke Protocol.
Continue reading "Editing Validation Performance Qualification Template into a Protocol."
Jul 15, 2023
HOW TO ENSURE YOUR REGULATORY REQUIREMENTS ARE cGMP COMPLIANT.
Regulatory requirements are easy to comply with when professionally produced protocol templates are used throughout a qualification project.
Continue reading "HOW TO ENSURE YOUR REGULATORY REQUIREMENTS ARE cGMP COMPLIANT."
Jul 14, 2023
Facilities HVAC Editing Template into Protocol.
Facilities HVAC validation tasks are greatly simplified when professional templates are used, Simply follow integral SOP and produce your own bespoke protocol.
Continue reading "Facilities HVAC Editing Template into Protocol."
Jul 14, 2023
Validation Templates | FDA | MHRA | WHO | EU | IQ | OQ | PQ | 4Q |
Validation Online was the 1st company to supply industry standard protocol templates for cGMP use and that was over 25 years ago & 24k templates ago.
Continue reading "Validation Templates | FDA | MHRA | WHO | EU | IQ | OQ | PQ | 4Q |"
Jul 14, 2023
Validation executables | FDA | EU | WHO | cGMP | FVLC | SOP's | QbD |
Validation is the verification of a related group of specifications.
Continue reading "Validation executables | FDA | EU | WHO | cGMP | FVLC | SOP's | QbD |"
Jul 14, 2023
Validation 4u | FDA | EU | WHO | GMP | QbD | FLCV | SOP's
Validation 4u methodology is the most direct route to cGMP compliance available - Use known quality interactive templates and get it right every time.
Continue reading "Validation 4u | FDA | EU | WHO | GMP | QbD | FLCV | SOP's "
Jul 13, 2023
Risk Based Validation | How to avoid Life Cycle Validation Costs |
Risk Based Validation is absolutely essential for cost effective validation & defining validation is often a very critical aspect of regulatory approval..
Continue reading "Risk Based Validation | How to avoid Life Cycle Validation Costs | "
Jul 12, 2023
How to write a superb Process Validation protocol
Understanding how to write a superb process validation template is fully explained in the SOP built in to each of our templates.
Continue reading "How to write a superb Process Validation protocol "
Jul 12, 2023
How to write a superb Cold Chain Validation protocol
Understanding how to write a superb Cold Chain Validation protocol is fully explained in the integral SOP built in to each of our templates.
Continue reading "How to write a superb Cold Chain Validation protocol "
Jul 12, 2023
How to write a superb Spreadsheet Validator
Understanding how to write a superb Spreadsheet Validator protocol is fully explained in the integral SOP built in to each of our templates
Continue reading "How to write a superb Spreadsheet Validator "
Jul 09, 2023
Validation Documentation Matrix | FDA | WHO | EU | GMP | QbD | FLCV |
Validation Documentation Matrix is a reviewable indicator. It must portray the cGMP state of all mandated documents in your validation task.
Continue reading "Validation Documentation Matrix | FDA | WHO | EU | GMP | QbD | FLCV |"
Jul 09, 2023
Validation Protocol Standards | FDA | EC | WHO | Pharma | Medical.....
Validation Protocol standards of wording must be accurate & unambiguous to ensure that executing a 4Q protocol produces the tangible evidence demanded
Continue reading "Validation Protocol Standards | FDA | EC | WHO | Pharma | Medical....."
Jul 09, 2023
Validation Quality Plan | FDA | MHRA | WHO | cGMP | EU | SOP's |
Attention to detail is required in preparing a quality validation plan. Time spent in getting the detail, scope, responsibilities and boundaries properly defined and documented ensures that dreadful blunders are unlikely to appear late in the task execution.
Continue reading "Validation Quality Plan | FDA | MHRA | WHO | cGMP | EU | SOP's | "
Jul 09, 2023
Validation4u | FDA | MHRA | WHO | EU | FLCV | cGMP | GxP | SOP's
Learn how validation4u; by way of tangible evidence can establish that an entity or a system conforms precisely to the cGMP rules and regulations.
Continue reading "Validation4u | FDA | MHRA | WHO | EU | FLCV | cGMP | GxP | SOP's"
Jul 09, 2023
Computer Vendor Audit | FDA | MHRA | WHO | EU | GAMP | SOP |
Computer Vendor Audit. FDA and GMP requirements in the USA & Associated Markets
Continue reading "Computer Vendor Audit | FDA | MHRA | WHO | EU | GAMP | SOP |"
Jul 07, 2023
How to write a superb Software Qualification
Understanding how to write a superb Software Qualification protocol is fully explained in the interactive integral SOP built in to each of our templates.
Continue reading "How to write a superb Software Qualification"
Jul 07, 2023
How to write a superb Operational Qualification.
Understanding how to write a superb Operational Qualification protocol is fully explained in the integral SOP built in to each template.
Continue reading "How to write a superb Operational Qualification."
Jul 01, 2023
How to write a superb Equipment Qualification
Understanding how to write a superb Equipment Qualification protocol is fully explained in the integral SOP built in to to each template.
Continue reading "How to write a superb Equipment Qualification"
Aug 10, 2022
Editing Process Qualification template into Protocol.
Process Qualification template used to run batches and check quality. Today the emphasis is on continuous product monitoring. tain products.
Continue reading "Editing Process Qualification template into Protocol. "
News for Today