HOW TO COMPLY WITH cGMP CALIBRATION REQUIREMENTS.
GMP Calibration requirements.

All applicable GMP calibration requirements should be encapsulated within a company approved Calibration Management Practices and Procedures manual. Routine and snap quality
audits must be used to confirm past, present and anticipate future compliance. The historic records establish with a calibration manager program are essential in facilitating accurate predictive forecasting of any instrument error propagation.
GMP regulated companies often use contract laboratories to satisfy their cGMP calibration reference their measurement and test equipment. If this is the case, FDA views the contract laboratory as an extension of the manufacturer's GMP program or quality system.
Normally FDA does not inspect contract laboratory facilities, but it does expect the manufacturer to audit the contract lab to verify that proper procedures are being used. Generally, the manufacturer of the finished device is responsible for assuring that all GMP calibration requirements are being complied with. This puts the onus on any auditor to ask about validation and calibration methodologies, techniques and standards used.
Calibration of Sensors

21 CFR Part 820 and 210 mandates that where the accuracy of instruments and or instrument sensors is judged as critical to the final efficacy, quality and or safety of a regulated product or could prove injurious to the integrity of any associated predicated data or data records; the individual instrument must be considered as a, “Critical Instrument”. Critical instruments must be entered into a planned calibration program and subjected to routine calibration against master instruments traceable to national standards, at a periodicity that has a written justification. They must be placarded with a “Calibrated Label”. This SOP lists, details and defines each of the 11 essential elements of cGMP compliant calibration and calibration management. It includes sample document formats, calibration labels layouts and details the importance of the post calibration report. The SOP concludes with a fully detailed; ready to use, Calibration Manager Auditor document. This is your complete calibration SOP to purchase and use.
International Measuring Instruments Calibration.

ISO
9001:2000, requirements must be incorporated into each manufacturer's measuring instruments manager program and form the basis of its quality controlling activities and used to ensure that all calibration delinquencies are removed from existing procedures. A good program can do more than reduce scrap rates. It can reduce expenditures for measurement instruments and related services, add to the confidence and expertise of the company’s staff,
and increase the customer's perception of company performance. A good program can protect the company from both routine and more subtle calibration delinquencies and provide records of internationally standardized metrology that would be hard to dispute.
Measuring Instruments Calibration Procedures.
Measurement instruments and tools differ greatly in construction and intent, and the specific activities necessary to calibrate these tools are just as varied. An understanding of the operation of each tool allows problems to be detected, adjustments to be made properly, and condition of tools to be assessed. These technical details are found in all good metrology procedures.
These procedures, to be effective, have to be valid to the tool that will be calibrated. It is very important for a manufacturer to design its metrology procedures around the specific tools and how they will be used to ensure calibration delinquencies do not arise.
There are many sources of historically validated metrology procedures for dimensional metrology tools.
The manufacturers of dimensional metrology tools can also be a great source of valid, proven measuring instruments. Metrology laboratories used for these services may be willing to share their procedures.
Staff experience can also be an excellent source of data on measuring instruments calibration manager procedures.
Traceability
Traceability of a measurement unit is really maintained by a dual chain of metrology that goes back to the international standard. Each comparison is accompanied by an estimation of measurement uncertainty. Measurement uncertainty is calculated through an internationally standardized discipline.
The requirements for measuring instruments calibration manager documented procedures for the control of measurement tools can be found in section 7.6 of ISO 9001:2000. For insight into this section, it is useful to refer to the manufacturing centered 1994 version of ISO 9000. Section 4.11, Control of Inspection, Measuring, and Test Equipment states that measuring equipment is used in a manner that ensures that the measurement uncertainty is known and is consistent with the required measurement capability. The intent of the requirements in both versions is the same. In-house calibrations that are not accompanied by calibration delinquencies have broken the chain of traceability.
Management

An GMP calibration requirements management system strives to attain
customer satisfaction through maintaining all sensory systems used in
the manufacturing and testing of a regulatory controlled product
operating within their design specification.
The management of GMP calibration requirements management system is
accomplished in the same way as management of the other processes
identified in the manufacturer’s management system. This manager
program must also log calibration delinquencies that arise to allow
predictive analysis of any instrumentation errors.
Some manufacturers may choose to develop a system manual which contains
the process description, planned quality objectives, key indicators of
process performance and references to the standards and procedures in
use. This can become a very valuable document that serves as the
road-map to a dynamic and effective, GMP calibration requirements
solution.
Mandatory GMP Calibration Requirements.
1: There must be a documented justification for CI classification.
2: All CI’s must be calibrated prior to use.
3: There must be a documented justification for CI calibration periodicities.
4: Calibrate within design Specification.
5: Unique serial numbers log must be established for CI’s.
6: Critical parameters must be obtained from calibrated CI sensors.
7. Calibrated & Non calibrated sensors must be appropriately placarded.
8. Adjusters that affect calibration; must be sealed against illegal use.
9: Completed CR must be circulated to an approved list of recipients.
10. Software associated with CI ‘s must be validated appropriately.
11. Calibration Management system must regularly audited.
News for Today
10000130 GMP Calibration
Requirements Issue-3
$89.00
21 CFR Part 820 and 211 mandates that where the accuracy of instruments and or instrument sensors is judged as critical to the final efficacy, quality and or safety of a regulated productor could prove injurious to the integrity of any associated predicated data oror data records; the individual instrument must be considered as a "Critical Instrument" (CI). A calendar based re-calibration schedule must be established to ensure the accuracy of all such instrumentation is continually verified. Trend analysis techniques must be used to determine that the time between calibrations (TBC) chosen; ensures that recalibration is scheduled well before the accuracy of the instrument or sensor could jeopardize any predicated process parameters.
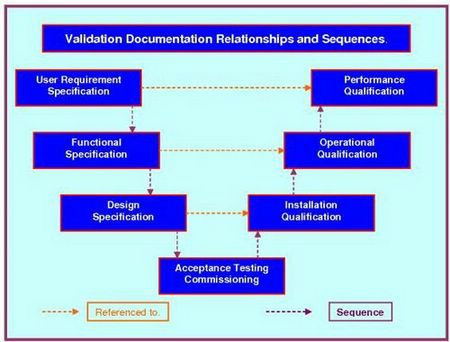
User Requirements Specification Template
$115.00
The User Requirements Specification template is the document that sets the standard, and specifies your requirements in a manner that ensures when a system or piece of equipment is selected for cGMP use that all essential support elements; i.e. maintenance, parts, operator & maintenance training, are planned and budgeted for. It uses three levels of User Requirements Specification Template (URS), URS Level 1, 2 and 3, and is the only URS to guarantee traceability from the URS through to the final OQ and PQ functionality testing. A requirement mandated by cGMP regulations. It can be used on mechanical, electrical and software controlled, monitored or driven systems.
Corporate Validation Manual $1,160.00
This definitive 1000 + page (including all attachments) Corporate Validation Manual arrives with you in USB memory stick format, this enables you at any time to download protocol or test-scrips documents and quickly edit them into company bespoke documents. In fact there are over $3,500.00 worth of superb documents, that form attachments to the DVM manual, which can be instantly copied. Once copied, the unique document interactive editing, allows you to produce high quality bespoke company documents; Such as the Validation Plan Template or the equally ubiquitous User requirements Specification template Design Qualification (DQ); in a few hours. The cost of the Definitive Validation Manual, will be recouped in the first few weeks of use. It will then go on to show a massive return on your original investment.