VALIDATION
Validation A Master Plan template is basically derived from the User Requirement Document (URS). When either management by policy; or an employee by cause; requests that a controlled process is instigated or an existing controlled process requires a serious modification (miner modification would be controlled under change control), a URS document is raised. Over the course of several meeting the URS is fleshed out to document all aspects of the requirement.
It is important that the document is properly scoped in order that the procurement, installation, commissioning, validation A, user training, maintenance, calibration and cleaning tasks are all investigated and defined adequately.
VMP is an acronym for “Validation Master Plan Template”: which is defined by the FDA as, establishing by objective evidence that all key aspects of the process equipment and ancillary system installation adhere to the manufacturer’s approved specification and that the recommendations of the equipment supplier have been suitably considered.
Validation Master Plan Template Rationale.
The Validation Master Plan Template (VMP); sometimes termed Master Validation A Plan (MVP) is used to display or present an overall picture to visiting auditors, of how the company has integrated cGMP into its day to day activities.
These auditors have not mandated a strict format for the VMP to follow; however, they have projected an expectation that they expect to be able to review such a document and that such a document must project a picture of how your company Validation Master Plan Template, has integrated ”current Good Manufacturing Practices” (cGMP); as promulgated in 21 CFR Part 820 & 211, into all aspects of the manufacture of a regulatory controlled product.
Even although the Validation A Master Plan Template is not a mandated document; it inevitably will be the first document regulators will ask to review. This is because they expect this document to clearly and concisely illustrate to them how management has delegated responsibilities, designed product processes, planned resources usage and established a fully trained competent work force. They will look for evidence to convince them that there is sufficient ongoing training to maintain these standards and sufficient auditing to prove it.
Validation Master Plan Compliance.
In authoring the Validation A Master Plan template extremely important commitments and decisions have to be made. Program conceptions have to be mated to the User Requirements Specifications (URS), Level 1, 2 and or 3, these specifications have to be mated to the VP or VMP. From these plans the User Requirements Specification (URS), the Validation A Risk Assessment (VRA), the Design Qualification (DQ), the Installation Qualification (IQ), the Operational Qualification (OQ), and the Performance Qualifications (PQ) have to be authorized, authored, approved for content, and issued for execution. The completed documentation has to be reviewed and accepted as complete by persons authorised to execute this role. All of these functions must be detailed in the Validation A Master Plan template, when the project concept demands that a VMP is required, or the Validation Plan (VP) where it does not. Responsibilities have to be declared, people have to be nominated, and everyone involved is duly served with a copy that carries the full authority of the company.
PLEASE CLICK HERE AND GO TO OUR STORE.
Validation Master Plan Techniques.
The Validation Master Plan template (VMP) must present an overall picture of the company facility, organization and capability. It must give a clear and concise overview of how the company has integrated all applicable current Good Manufacturing Practice (cGMP) requirements into its operations. It must define validation A activities and allot responsibilities for authoring, reviewing, approving, and executing validation A documentation and tasks. The Validation Master Plan template must comply with all the appropriate requirements documented in 21 Code of Federal Regulation Part 11, 210, 211 and 820 legislation.
Validation Master Plan Template Derivation.
Validation Master Plan Guidance.
The Validation Master Plan template is a top layer document and should not go into specific detail; but present an overall picture of the company facility, organization and capability. It must give a clear and concise overview, to a reviewer, of how the company has integrated all the applicable cGMP requirements into every aspect of its operations. It must define validation activities and allot responsibilities for authoring, reviewing, approving, and executing validation A documentation and tasks.
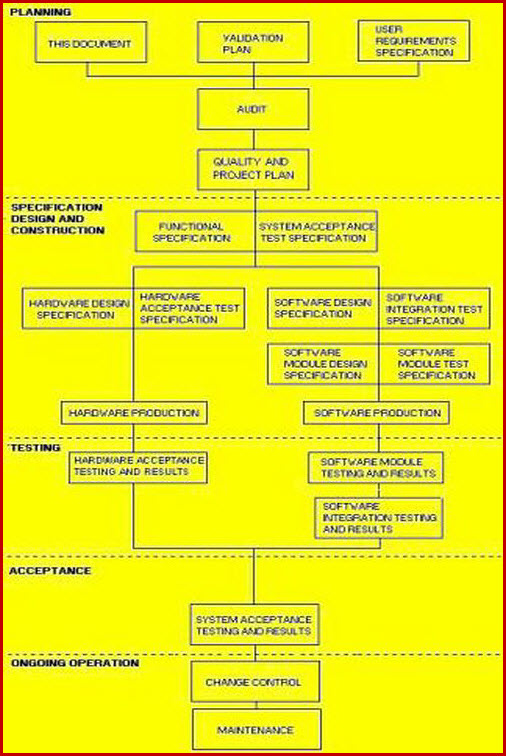
The Validation A master plan template must define the range of documentation spreading from the Validation A Master Plan template to the VP, URS, DQ, IQ, OQ, P1Q, and P2Q. It must explain and detail the company’s approach to risk based validation A and the interaction of the VRA, Master Plan template, and 21 CFR Part 11.
Facilities are portrayed with the use of layered drawings; where different layers show individual systems and equipment lists give equipment type and identity details. It is normal to include layered drawings to enable a clear and easily observed presentation of the following systems.
1) Facility building overall location and access.
2) Facility production/clerical/storage/controlled areas, rooms or zones.
3) Raw material ingress and finished product egress routes.
4) Personnel ingress and egress routes, along with changing areas.
5) Utility Layouts
6) Electrical layouts
7) Controlled areas along with air flow directions and pressure regimes.
8) Dressing codes for these controlled areas.
Risk Assessment and VMP
Since we are all operating validation which we deem to be "Risk Based" we are required to ensure that the Validation Master Plan template (VMP) dutifully ensures that all validation activities mandated in the VMP are assessed by the execution of a Validation Risk Assessment (VRA) protocol.
The VRA is fast becoming the most important document in the validation train. The VRA reassures the regulators that you have looked at specific equipment functionality and considered the appropriate level of validation that is required. You have also considered various aspects of its use and the implications of any malfunctions. From the results of this exercise the scope of all validation activity can and must be justified. This Validation Master Plan template is a robust and simple document to execute, one that will lead you through the process and deliver a result that can be used as the foundation for your validation activities.
This VRA now includes the assessment table for categorizing and documenting the new 21 CFR Part 11 guidance ruling on what predicate data must be stored in a Part compliant system, along with the new broadsheet to establish your new database of part 11 records. (now mandatory).
News for Today
Validation Master Plan Template (Issue 8) -- $115.00
All you need to do is follow the prompts in the attached SOP. They will take you through the completion process section, by section. At the end of this process your generic document has progressed into a detailed, referenced, bespoke company Validation Master Plan Template. The document follows our three level URS system that ensures functionality traceability from the URS to the various testing protocols. A great document to author and use. This document interfaces with our Validation Online Risk Assessment (VRA), Validation Online Project Plan (VP), User Requirements Specification (URS), giving a seamless flow from your VMP through the VP - IQ - OQ - PQ, while integrating flawlessly with the URS - DQ - VRA.
Validation Plan (Issue 10) -- $93.00
The Validation Plan (VP), is the starting point for any validation task, and the most important validation Online document. It improves validation efficiency greatly by forcing all concerned to document, review, and discuss, the proposed methods and allotted responsibilities. It is a mandated; referenced in the Validation Master Plan and detail document.
While in the past validation was more focused on functions of procedures, recently the focus has progressed into infrastructure, networked systems and on security, authenticity and integrity of data acquired and evaluated by systems.
User Requirements Specification Template
$115.00
The User Requirements Specification template is the document that sets the standard, and specifies your requirements in a manner that ensures when a system or piece of equipment is selected for cGMP use that all essential support elements; i.e. maintenance, parts, operator & maintenance training, are planned and budgeted for. It uses three levels of User Requirements Specification Template (URS), URS Level 1, 2 and 3, and is the only URS to guarantee traceability from the URS through to the final OQ and PQ functionality testing. A requirement mandated by cGMP regulations. It can be used on mechanical, electrical and software controlled, monitored or driven systems.
Corporate Validation Manual $1,160.00
This definitive 1000 + page (including all attachments) Corporate Validation Manual arrives with you in USB memory stick format, this enables you at any time to download protocol or test-scrips documents and quickly edit them into company bespoke documents. In fact there are over $3,500.00 worth of superb documents, that form attachments to the DVM manual, which can be instantly copied. Once copied, the unique document interactive editing, allows you to produce high quality bespoke company documents; Such as the Validation Plan Template or the equally ubiquitous User requirements Specification template Design Qualification (DQ); in a few hours. The cost of the Definitive Validation Manual, will be recouped in the first few weeks of use. It will then go on to show a massive return on your original investment.